GPR109A mediates the effects of hippuric acid on regulating osteoclastogenesis and bone resorption in mice
- PMID: 33420329
- PMCID: PMC7794563
- DOI: 10.1038/s42003-020-01564-2
GPR109A mediates the effects of hippuric acid on regulating osteoclastogenesis and bone resorption in mice
Abstract
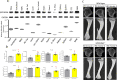
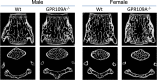
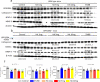
The G protein-coupled receptor 109 A (GPR109A) is robustly expressed in osteoclastic precursor macrophages. Previous studies suggested that GPR109A mediates effects of diet-derived phenolic acids such as hippuric acid (HA) and 3-(3-hydroxyphenyl) propionic acid (3-3-PPA) on promoting bone formation. However, the role of GPR109A in metabolic bone homeostasis and osteoclast differentiation has not been investigated. Using densitometric, bone histologic and molecular signaling analytic methods, we uncovered that bone mass and strength were significantly higher in tibia and spine of standard rodent diet weaned 4-week-old and 6-month-old GPR109A gene deletion (GPR109A-/-) mice, compared to their wild type controls. Osteoclast numbers in bone and in ex vivo bone marrow cell cultures were significantly decreased in GPR109A-/- mice compared to wild type controls. In accordance with these data, CTX-1 in bone marrow plasma and gene expression of bone resorption markers (TNFα, TRAP, Cathepsin K) were significantly decreased in GPR109A-/- mice, while on the other hand, P1NP was increased in serum from both male and female GPR109A-/- mice compared to their respective controls. GPR109A deletion led to suppressed Wnt/β-catenin signaling in osteoclast precursors to inhibit osteoclast differentiation and activity. Indeed, HA and 3-3-PPA substantially inhibited RANKL-induced GPR109A expression and Wnt/β-catenin signaling in osteoclast precursors and osteoclast differentiation. Resultantly, HA significantly inhibited bone resorption and increased bone mass in wild type mice, but had no additional effects on bone in GPR109A-/- mice compared with their respective untreated control mice. These results suggest an important role for GPR109A during osteoclast differentiation and bone resorption mediating effects of HA and 3-3-PPA on inhibiting bone resorption during skeletal development.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Hippuric acid and 3-(3-hydroxyphenyl) propionic acid inhibit murine osteoclastogenesis through RANKL-RANK independent pathway.J Cell Physiol. 2020 Jan;235(1):599-610. doi: 10.1002/jcp.28998. Epub 2019 Jul 4. J Cell Physiol. 2020. PMID: 31271661 Free PMC article.
-
GPR109A gene deletion ameliorates gonadectomy-induced bone loss in mice.Bone. 2022 Aug;161:116422. doi: 10.1016/j.bone.2022.116422. Epub 2022 Apr 27. Bone. 2022. PMID: 35489706
-
Exploring GPR109A Receptor Interaction with Hippuric Acid Using MD Simulations and CD Spectroscopy.Int J Mol Sci. 2022 Nov 26;23(23):14778. doi: 10.3390/ijms232314778. Int J Mol Sci. 2022. PMID: 36499106 Free PMC article.
-
The fungal metabolite (+)-terrein abrogates osteoclast differentiation via suppression of the RANKL signaling pathway through NFATc1.Int Immunopharmacol. 2020 Jun;83:106429. doi: 10.1016/j.intimp.2020.106429. Epub 2020 Mar 26. Int Immunopharmacol. 2020. PMID: 32222639
-
GPR120 Inhibits RANKL-Induced Osteoclast Formation and Resorption by Attenuating Reactive Oxygen Species Production in RAW264.7 Murine Macrophages.Int J Mol Sci. 2021 Sep 29;22(19):10544. doi: 10.3390/ijms221910544. Int J Mol Sci. 2021. PMID: 34638884 Free PMC article.
Cited by
-
The gut-derived metabolites as mediators of the effect of healthy nutrition on the brain.Front Nutr. 2023 Jun 9;10:1155533. doi: 10.3389/fnut.2023.1155533. eCollection 2023. Front Nutr. 2023. PMID: 37360297 Free PMC article. Review.
-
The fecal microbiota of patients with primary biliary cholangitis (PBC) causes PBC-like liver lesions in mice and exacerbates liver damage in a mouse model of PBC.Gut Microbes. 2024 Jan-Dec;16(1):2383353. doi: 10.1080/19490976.2024.2383353. Epub 2024 Aug 6. Gut Microbes. 2024. PMID: 39105259 Free PMC article.
-
The survival of B cells is compromised in kidney disease.Nat Commun. 2024 Dec 30;15(1):10842. doi: 10.1038/s41467-024-55187-w. Nat Commun. 2024. PMID: 39738044 Free PMC article.
-
Unraveling Protein-Metabolite Interactions in Precision Nutrition: A Case Study of Blueberry-Derived Metabolites Using Advanced Computational Methods.Metabolites. 2024 Aug 3;14(8):430. doi: 10.3390/metabo14080430. Metabolites. 2024. PMID: 39195526 Free PMC article.
-
Fructooligosaccharides act on the gut-bone axis to improve bone independent of Tregs and alter osteocytes in young adult C57BL/6 female mice.JBMR Plus. 2024 Feb 21;8(5):ziae021. doi: 10.1093/jbmrpl/ziae021. eCollection 2024 May. JBMR Plus. 2024. PMID: 38562914 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

