Voltage-independent GluN2A-type NMDA receptor Ca2+ signaling promotes audiogenic seizures, attentional and cognitive deficits in mice
- PMID: 33420383
- PMCID: PMC7794508
- DOI: 10.1038/s42003-020-01538-4
Voltage-independent GluN2A-type NMDA receptor Ca2+ signaling promotes audiogenic seizures, attentional and cognitive deficits in mice
Erratum in
-
Author Correction: Voltage-independent GluN2A-type NMDA receptor Ca2+ signaling promotes audiogenic seizures, attentional and cognitive deficits in mice.Commun Biol. 2021 Feb 9;4(1):187. doi: 10.1038/s42003-021-01717-x. Commun Biol. 2021. PMID: 33564096 Free PMC article. No abstract available.
Abstract
The NMDA receptor-mediated Ca2+ signaling during simultaneous pre- and postsynaptic activity is critically involved in synaptic plasticity and thus has a key role in the nervous system. In GRIN2-variant patients alterations of this coincidence detection provoked complex clinical phenotypes, ranging from reduced muscle strength to epileptic seizures and intellectual disability. By using our gene-targeted mouse line (Grin2aN615S), we show that voltage-independent glutamate-gated signaling of GluN2A-containing NMDA receptors is associated with NMDAR-dependent audiogenic seizures due to hyperexcitable midbrain circuits. In contrast, the NMDAR antagonist MK-801-induced c-Fos expression is reduced in the hippocampus. Likewise, the synchronization of theta- and gamma oscillatory activity is lowered during exploration, demonstrating reduced hippocampal activity. This is associated with exploratory hyperactivity and aberrantly increased and dysregulated levels of attention that can interfere with associative learning, in particular when relevant cues and reward outcomes are disconnected in space and time. Together, our findings provide (i) experimental evidence that the inherent voltage-dependent Ca2+ signaling of NMDA receptors is essential for maintaining appropriate responses to sensory stimuli and (ii) a mechanistic explanation for the neurological manifestations seen in the NMDAR-related human disorders with GRIN2 variant-meidiated intellectual disability and focal epilepsy.
Conflict of interest statement
The authors declare no competing interests.
Figures
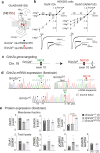
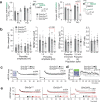





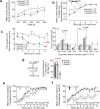
References
-
- Hofer, M. & Constantine-Paton, M. in Progress in Brain Research Vol. 102 (eds. Van Pelt, J., Corner, M. A., Uylings, H. B. M. & Lopes Da Silva, F. H.) 277–285 (Elsevier, 1994).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

