Streamlined copper defenses make Bordetella pertussis reliant on custom-made operon
- PMID: 33420409
- PMCID: PMC7794356
- DOI: 10.1038/s42003-020-01580-2
Streamlined copper defenses make Bordetella pertussis reliant on custom-made operon
Abstract
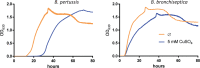
Copper is both essential and toxic to living beings, which tightly controls its intracellular concentration. At the host-pathogen interface, copper is used by phagocytic cells to kill invading microorganisms. We investigated copper homeostasis in Bordetella pertussis, which lives in the human respiratory mucosa and has no environmental reservoir. B. pertussis has considerably streamlined copper homeostasis mechanisms relative to other Gram-negative bacteria. Its single remaining defense line consists of a metallochaperone diverted for copper passivation, CopZ, and two peroxide detoxification enzymes, PrxGrx and GorB, which together fight stresses encountered in phagocytic cells. Those proteins are encoded by an original, composite operon assembled in an environmental ancestor, which is under sensitive control by copper. This system appears to contribute to persistent infection in the nasal cavity of B. pertussis-infected mice. Combining responses to co-occurring stresses in a tailored operon reveals a strategy adopted by a host-restricted pathogen to optimize survival at minimal energy expenditure.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Solioz, M. Copper and Bacteria. Evolution, homeostasis and toxicity, Springer Nature Switzerland AG, Cham, Switzerland (2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

