Neurexins: molecular codes for shaping neuronal synapses
- PMID: 33420412
- PMCID: PMC7612283
- DOI: 10.1038/s41583-020-00415-7
Neurexins: molecular codes for shaping neuronal synapses
Abstract
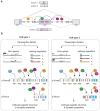
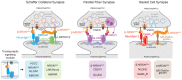
The function of neuronal circuits relies on the properties of individual neuronal cells and their synapses. We propose that a substantial degree of synapse formation and function is instructed by molecular codes resulting from transcriptional programmes. Recent studies on the Neurexin protein family and its ligands provide fundamental insight into how synapses are assembled and remodelled, how synaptic properties are specified and how single gene mutations associated with neurodevelopmental and psychiatric disorders might modify the operation of neuronal circuits and behaviour. In this Review, we first summarize insights into Neurexin function obtained from various model organisms. We then discuss the mechanisms and logic of the cell type-specific regulation of Neurexin isoforms, in particular at the level of alternative mRNA splicing. Finally, we propose a conceptual framework for how combinations of synaptic protein isoforms act as 'senders' and 'readers' to instruct synapse formation and the acquisition of cell type-specific and synapse-specific functional properties.
Figures



References
-
- Hobert O. Terminal Selectors of Neuronal Identity. Curr Top Dev Biol. 2016;116:455–475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

