Efficacy of bevacizumab for vitreous haemorrhage in proliferative diabetic retinopathy with prior complete panretinal photocoagulation
- PMID: 33420422
- PMCID: PMC8526678
- DOI: 10.1038/s41433-020-01384-y
Efficacy of bevacizumab for vitreous haemorrhage in proliferative diabetic retinopathy with prior complete panretinal photocoagulation
Abstract
Purpose: To investigate the efficacy of intravitreal bevacizumab injections (IVBs) for vitreous haemorrhage (VH) in proliferative diabetic retinopathy (PDR) with prior complete panretinal photocoagulation (PRP).
Methods: A multicentre cohort study of eyes with new VH in PDR after documented previous complete PRP was performed. Eyes were grouped according to IVB treatment at baseline, and cumulative rate of vitrectomy and spontaneous clear-up rate were compared as the main outcome. Eyes requiring vitrectomy within 1 month, or with tractional retinal detachment (TRD), or with spontaneous clear-up within 1 month, were excluded.
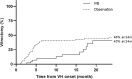
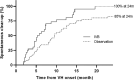
Results: In total, 44 eyes with IVB and 92 control eyes without IVB were followed up to 20.1 months. Cumulative probability of vitrectomy was lower in the IVB group at 12 months (0.16 vs 0.42, IVB vs controls), and throughout the follow-up period (p = 0.005). Cumulative probability of spontaneous clear-up was higher in the IVB group at 12 months (0.81 vs 0.68, IVB vs controls), and throughout the follow-up period (p = 0.013). Best-corrected visual acuity (BCVA) at 1 month after onset of VH was significantly better in the IVB group (0.513 vs 0.942 logarithm of the minimal angle of resolution, p = 0.002); however, the difference of BCVA lost significance with further follow-up. IVB treatment was the only factor significantly associated with vitrectomy risk on multivariate analysis (p = 0.047, hazard ratio 0.506).
Conclusion: In VH after prior complete PRP, IVB was effective in decreasing vitrectomy requirement, although overall visual benefit was short-term. IVB can be considered to defer vitrectomy in PDR VH eyes with prior complete PRP and no TRD.
© 2021. The Author(s), under exclusive licence to The Author(s), under exclusive licence to The Royal College of Ophthalmologists.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

