Empagliflozin and health-related quality of life outcomes in patients with heart failure with reduced ejection fraction: the EMPEROR-Reduced trial
- PMID: 33420498
- PMCID: PMC8014525
- DOI: 10.1093/eurheartj/ehaa1007
Empagliflozin and health-related quality of life outcomes in patients with heart failure with reduced ejection fraction: the EMPEROR-Reduced trial
Abstract
Aims: In this secondary analysis of the EMPEROR-Reduced trial, we sought to evaluate whether the benefits of empagliflozin varied by baseline health status and how empagliflozin impacted patient-reported outcomes in patients with heart failure with reduced ejection fraction.
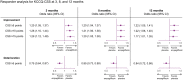
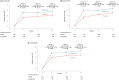
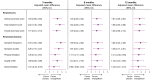
Methods and results: Health status was assessed by the Kansas City Cardiomyopathy Questionnaires-clinical summary score (KCCQ-CSS). The influence of baseline KCCQ-CSS (analyzed by tertiles) on the effect of empagliflozin on major outcomes was examined using Cox proportional hazards models. Responder analyses were performed to assess the odds of improvement and deterioration in KCCQ scores related to treatment with empagliflozin. Empagliflozin reduced the primary outcome of cardiovascular death or heart failure hospitalization regardless of baseline KCCQ-CSS tertiles [hazard ratio (HR) 0.83 (0.68-1.02), HR 0.74 (0.58-0.94), and HR 0.61 (0.46-0.82) for <62.5, 62.6-85.4, and ≥85.4 score tertiles, respectively; P-trend = 0.10]. Empagliflozin improved KCCQ-CSS, total symptom score, and overall summary score at 3, 8, and 12 months. More patients on empagliflozin had ≥5-point [odds ratio (OR) 1.20 (1.05-1.37)], 10-point [OR 1.26 (1.10-1.44)], and 15-point [OR 1.29 (1.12-1.48)] improvement and fewer had ≥5-point [OR 0.75 (0.64-0.87)] deterioration in KCCQ-CSS at 3 months. These benefits were sustained at 8 and 12 months and were similar for other KCCQ domains.
Conclusion: Empagliflozin improved cardiovascular death or heart failure hospitalization risk across the range of baseline health status. Empagliflozin improved health status across various domains, and this benefit was sustained during long-term follow-up.
Clinical trial registration: URL: https://www.clinicaltrials.gov. Unique identifier: NCT03057977.
Keywords: Empagliflozin; Health status; Heart failure; Quality of life; SGLT2 inhibitors.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures


Comment in
-
Quality of life in EMPEROR-Reduced: emphasizing what is important to patients while identifying strategies to support more patient-centred care.Eur Heart J. 2021 Mar 31;42(13):1213-1215. doi: 10.1093/eurheartj/ehab057. Eur Heart J. 2021. PMID: 33595088 No abstract available.
References
-
- Lewis EF. Assessing the impact of heart failure therapeutics on quality of life and functional capacity. Curr Treat Options Cardiovasc Med 2013;15:425–436. - PubMed
-
- Vaishnava P, Lewis EF. Assessment of quality of life in severe heart failure. Curr Heart Fail Rep 2007;4:170–177. - PubMed
-
- Clinical Outcome Assessments (COA) Qualification Submissions Office of Cardiology Hematology, Endocrinology, and Nephrology (OCEHM) Division of Cardiovascular and Nephrology (DCN). DDT COA #000084: Kansas City Cardiomyopathy Questionnaire (KCCQ). 2020. https://www.fda.gov/drugs/clinical-outcome-assessment-coa-qualification-....
-
- US-FDA. Treatment for Heart Failure: Endpoints for Drug Development Guidance for Industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents....
-
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang C-E, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde A-M; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

