Pilot trial of high-dose vitamin C in critically ill COVID-19 patients
- PMID: 33420963
- PMCID: PMC7794643
- DOI: 10.1186/s13613-020-00792-3
Pilot trial of high-dose vitamin C in critically ill COVID-19 patients
Abstract
Background: Few specific medications have been proven effective for the treatment of patients with severe coronavirus disease 2019 (COVID-19). Here, we tested whether high-dose vitamin C infusion was effective for severe COVID-19.
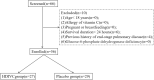
Methods: This randomized, controlled, clinical trial was performed at 3 hospitals in Hubei, China. Patients with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the ICU were randomly assigned in as 1:1 ratio to either the high-dose intravenous vitamin C (HDIVC) or the placebo. HDIVC group received 12 g of vitamin C/50 ml every 12 h for 7 days at a rate of 12 ml/hour, and the placebo group received bacteriostatic water for injection in the same way within 48 h of arrival to ICU. The primary outcome was invasive mechanical ventilation-free days in 28 days (IMVFD28). Secondary outcomes were 28-day mortality, organ failure (Sequential Organ Failure Assessment (SOFA) score), and inflammation progression (interleukin-6).
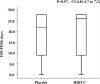
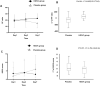
Results: Only 56 critical COVID-19 patients were ultimately recruited due to the early control of the outbreak. There was no difference in IMVFD28 between two groups (26.0 [9.0-28.0] in HDIVC vs 22.0 [8.50-28.0] in control, p = 0.57). HDIVC failed to reduce 28-day mortality (P = 0.27). During the 7-day treatment period, patients in the HDIVC group had a steady rise in the PaO2/FiO2 (day 7: 229 vs. 151 mmHg, 95% CI 33 to 122, P = 0.01), which was not observed in the control group. IL-6 in the HDIVC group was lower than that in the control group (19.42 vs. 158.00; 95% CI -301.72 to -29.79; P = 0.04) on day 7.
Conclusion: This pilot trial showed that HDIVC failed to improve IMVFD28, but might show a potential signal of benefit in oxygenation for critically ill patients with COVID-19 improving PaO2/FiO2 even though.
Keywords: Coronavirus disease 2019; High-dose intravenous vitamin C; Severe acute respiratory syndrome coronavirus 2.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in china - key questions for impact assessment. N Engl J Med. 2020;382(8):692–694. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

