Western Blotting Using In-Gel Protein Labeling as a Normalization Control: Advantages of Stain-Free Technology
- PMID: 33421007
- PMCID: PMC12288770
- DOI: 10.1007/978-1-0716-1186-9_28
Western Blotting Using In-Gel Protein Labeling as a Normalization Control: Advantages of Stain-Free Technology
Abstract
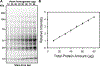
Western blotting is one of the most used techniques in research laboratories. It is popular because it is an easy way of semiquantifying protein amounts in different samples. In Western blotting, the most commonly used method for controlling the differences in the amount of protein loaded is to independently quantify housekeeping proteins (typically actin, GAPDH or tubulin). Another less commonly used method is total protein normalization using stains, such as Ponceau S or Coomassie Brilliant Blue, which stains all the proteins on the blots. A less commonly used but powerful total protein staining technique is stain-free normalization. The stain-free technology is able to detect total protein in a large linear dynamic range and has the advantage of allowing protein detection on the gel before transblotting. This chapter discusses the theory, advantages, and method used to do total protein quantification using stain-free gels for normalization of Western blots.
Keywords: Immunoblotting; Loading control; Stain-free technology; Total protein normalization; Western blotting.
Figures

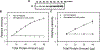
References
-
- Hu X, Du S, Yu J, Yang X, Yang C, Zhou D, Wang Q, Qin S, Yan X, He L, Han D, Wan C (2016) Common housekeeping proteins are upregulated in colorectal adenocarcinoma and hepatocellular carcinoma, making the total protein a better “housekeeper”. Oncotarget 7 (41):66679–66688. doi: 10.18632/oncotarget.11439 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

