Expression of GFAP and Tau Following Blast Exposure in the Cerebral Cortex of Ferrets
- PMID: 33421075
- PMCID: PMC8453607
- DOI: 10.1093/jnen/nlaa157
Expression of GFAP and Tau Following Blast Exposure in the Cerebral Cortex of Ferrets
Abstract
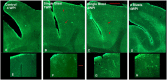
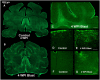
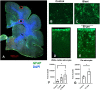
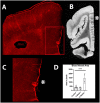
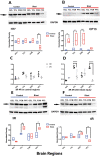
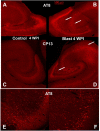
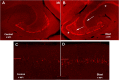
Blast exposures are a hallmark of contemporary military conflicts. We need improved preclinical models of blast traumatic brain injury for translation of pharmaceutical and therapeutic protocols. Compared with rodents, the ferret brain is larger, has substantial sulci, gyri, a higher white to gray matter ratio, and the hippocampus in a ventral position; these attributes facilitate comparison with the human brain. In this study, ferrets received compressed air shock waves and subsequent evaluation of glia and forms of tau following survival of up to 12 weeks. Immunohistochemistry and Western blot demonstrated altered distributions of astrogliosis and tau expression after blast exposure. Many aspects of the astrogliosis corresponded to human pathology: increased subpial reactivity, gliosis at gray-white matter interfaces, and extensive outlining of blood vessels. MRI analysis showed numerous hypointensities occurring in the 12-week survival animals, appearing to correspond to luminal expansions of blood vessels. Changes in forms of tau, including phosphorylated tau, and the isoforms 3R and 4R were noted using immunohistochemistry and Western blot in specific regions of the cerebral cortex. Of particular interest were the 3R and 4R isoforms, which modified their ratio after blast. Our data strongly support the ferret as an animal model with highly translational features to study blast injury.
Keywords: Aquaporin; Astrocyte; Blood vessels; Phosphorylated tau; Pia; Tau isoforms.
2021 American Association of Neuropathologists, Inc. This work is written by US Government employees and is in the public domain in the US.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

