Lipocalin-2 mediates the rejection of neural transplants
- PMID: 33421207
- PMCID: PMC12315500
- DOI: 10.1096/fj.202001018R
Lipocalin-2 mediates the rejection of neural transplants
Abstract
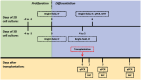
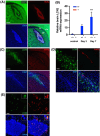
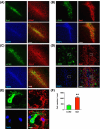
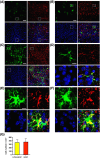
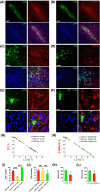
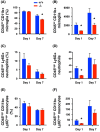
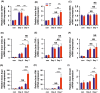
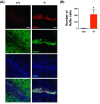
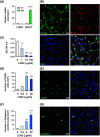
Lipocalin-2 (LCN2) has been implicated in promoting apoptosis and neuroinflammation in neurological disorders; however, its role in neural transplantation remains unknown. In this study, we cultured and differentiated Lund human mesencephalic (LUHMES) cells into human dopaminergic-like neurons and found that LCN2 mRNA was progressively induced in mouse brain after the intrastriatal transplantation of human dopaminergic-like neurons. The induction of LCN2 protein was detected in a subset of astrocytes and neutrophils infiltrating the core of the engrafted sites, but not in neurons and microglia. LCN2-immunoreactive astrocytes within the engrafted sites expressed lower levels of A1 and A2 astrocytic markers. Recruitment of microglia, neutrophils, and monocytes after transplantation was attenuated in LCN2 deficiency mice. The expression of M2 microglial markers was significantly elevated and survival of engrafted neurons was markedly improved after transplantation in LCN2 deficiency mice. Brain type organic cation transporter (BOCT), the cell surface receptor for LCN2, was induced in dopaminergic-like neurons after differentiation, and treatment with recombinant LCN2 protein directly induced apoptosis in dopaminergic-like neurons in a dose-dependent manner. Our results, therefore, suggested that LCN2 is a neurotoxic factor for the engrafted neurons and a modulator of neuroinflammation. LCN2 inhibition may be useful in reducing rejection after neural transplantation.
Keywords: Lipocalin-2; graft; neuroinflammation; neutrophil gelatinase-associated lipocalin; rejection; transplantation.
© 2020 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care. 2015;21:s12‐s23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

