Protective effect of baicalin against pulmonary arterial hypertension vascular remodeling through regulation of TNF-α signaling pathway
- PMID: 33421306
- PMCID: PMC7796790
- DOI: 10.1002/prp2.703
Protective effect of baicalin against pulmonary arterial hypertension vascular remodeling through regulation of TNF-α signaling pathway
Abstract
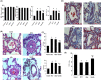
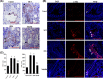
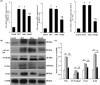
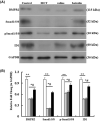
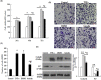
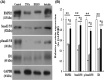
Pulmonary arterial hypertension (PAH) is a progressive cardiovascular disease with high mortality. However, there were no efficient medical drugs for PAH to enormously improve the survival and quality of life measures. The present study aimed to explore the protective effect of baicalin against experimental PAH in vivo and vitro. All the experimental rats received intraperitoneal injection of monocrotaline (MCT) to induce PAH model. Baicalin was given by intragastric administration from 2 days after MCT injection. Forty animals were randomly divided into four groups: Control, MCT, saline-, and baicalin-treated groups (n = 10 in each). Post-operation, hemodynamic data, and index of right ventricular hypertrophy (RVHI) were recorded to evaluate the inhibition of baicalin on MCT-induced PAH. Furthermore, pulmonary artery smooth muscle cells (PASMCs) model induced by tumor necrosis factor-α (TNF-α) was used to observe the inhibition of vascular cells proliferation in vitro. The results demonstrated that baicalin significantly attenuated MCT-induced right ventricular systolic pressure (RVSP), the index of right ventricular hypertrophy, and vessel wall thickness; inhibit inflammatory and cell proliferation induced by MCT or TNF-α, respectively. In addition, we found that baicalin might protect against experimental PAH via regulating the TNF-α/BMPR2 signaling pathway.
Keywords: BMPR2; Baicalin; PAH; TNF-α; Vascular remodeling.
© 2021 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
All authors declare no conflict of interest.
The authors declare that they have no competing interests.
Figures
Similar articles
-
C-C Motif chemokine receptor-2 blockade ameliorates pulmonary hypertension in rats and synergizes with a pulmonary vasodilator.Cardiovasc Res. 2025 Jul 8;121(7):1076-1090. doi: 10.1093/cvr/cvae244. Cardiovasc Res. 2025. PMID: 39556088 Free PMC article.
-
Protective effects of dioscin on vascular remodeling in pulmonary arterial hypertension via adjusting GRB2/ERK/PI3K-AKT signal.Biomed Pharmacother. 2021 Jan;133:111056. doi: 10.1016/j.biopha.2020.111056. Epub 2020 Nov 28. Biomed Pharmacother. 2021. PMID: 33378960
-
Berberine attenuates hypoxia-induced pulmonary arterial hypertension via bone morphogenetic protein and transforming growth factor-β signaling.J Cell Physiol. 2019 Aug;234(10):17482-17493. doi: 10.1002/jcp.28370. Epub 2019 Feb 20. J Cell Physiol. 2019. PMID: 30786011
-
Approaches to treat pulmonary arterial hypertension by targeting BMPR2: from cell membrane to nucleus.Cardiovasc Res. 2021 Sep 28;117(11):2309-2325. doi: 10.1093/cvr/cvaa350. Cardiovasc Res. 2021. PMID: 33399862 Free PMC article. Review.
-
The Role of Bone Morphogenetic Protein Receptor Type 2 (BMPR2) and the Prospects of Utilizing Induced Pluripotent Stem Cells (iPSCs) in Pulmonary Arterial Hypertension Disease Modeling.Cells. 2022 Nov 29;11(23):3823. doi: 10.3390/cells11233823. Cells. 2022. PMID: 36497082 Free PMC article. Review.
Cited by
-
Safflower Alleviates Pulmonary Arterial Hypertension by Inactivating NLRP3: A Combined Approach of Network Pharmacology and Experimental Verification.Clin Respir J. 2024 Aug;18(8):e13826. doi: 10.1111/crj.13826. Clin Respir J. 2024. PMID: 39155275 Free PMC article.
-
Novel insights into the potential applications of stem cells in pulmonary hypertension therapy.Respir Res. 2024 Jun 7;25(1):237. doi: 10.1186/s12931-024-02865-4. Respir Res. 2024. PMID: 38849894 Free PMC article. Review.
-
Unleashing the Potential of Nrf2: A Novel Therapeutic Target for Pulmonary Vascular Remodeling.Antioxidants (Basel). 2023 Nov 7;12(11):1978. doi: 10.3390/antiox12111978. Antioxidants (Basel). 2023. PMID: 38001831 Free PMC article. Review.
-
Natural Products for the Treatment of Pulmonary Hypertension: Mechanism, Progress, and Future Opportunities.Curr Issues Mol Biol. 2023 Mar 13;45(3):2351-2371. doi: 10.3390/cimb45030152. Curr Issues Mol Biol. 2023. PMID: 36975522 Free PMC article. Review.
-
Traditional Chinese medicine monomers: Targeting pulmonary artery smooth muscle cells proliferation to treat pulmonary hypertension.Heliyon. 2023 Mar 25;9(4):e14916. doi: 10.1016/j.heliyon.2023.e14916. eCollection 2023 Apr. Heliyon. 2023. PMID: 37128338 Free PMC article. Review.
References
-
- Lambert M, Capuano V, Boet A, et al. Characterization of kcnk3‐mutated rat, a novel model of pulmonary hypertension. Circ Res. 2019;125:678‐695. - PubMed
-
- de Man FS, Handoko ML, Vonk‐Noordegraaf A. The unknown pathophysiological relevance of right ventricular hypertrophy in pulmonary arterial hypertension. Eur Respir J. 2019;53:1900255. - PubMed
-
- Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen‐associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156‐163. - PubMed
-
- Kurakula K, Sun XQ, Happé C, et al. Prevention of progression of pulmonary hypertension by the Nur77 agonist 6‐mercaptopurine: role of BMP signaling. Eur Respir J. 2019;54:1802400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

