miR-10b-5p Rescues Diabetes and Gastrointestinal Dysmotility
- PMID: 33421511
- PMCID: PMC8532043
- DOI: 10.1053/j.gastro.2020.12.062
miR-10b-5p Rescues Diabetes and Gastrointestinal Dysmotility
Abstract
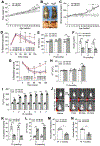
Background & aims: Interstitial cells of Cajal (ICCs) and pancreatic β cells require receptor tyrosine kinase (KIT) to develop and function properly. Degeneration of ICCs is linked to diabetic gastroparesis. The mechanisms linking diabetes and gastroparesis are unclear, but may involve microRNA (miRNA)-mediated post-transcriptional gene silencing in KIT+ cells.
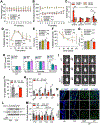
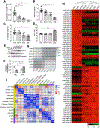
Methods: We performed miRNA-sequencing analysis from isolated ICCs in diabetic mice and plasma from patients with idiopathic and diabetic gastroparesis. miR-10b-5p target genes were identified and validated in mouse and human cell lines. For loss-of-function studies, we used KIT+ cell-restricted mir-10b knockout mice and KIT+ cell depletion mice. For gain-of-function studies, a synthetic miR-10b-5p mimic was injected in multiple diabetic mouse models. We compared the efficacy of miR-10b-5p mimic treatment vs antidiabetic and prokinetic medicines.
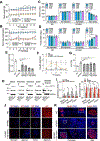
Results: miR-10b-5p is highly expressed in ICCs from healthy mice, but drastically depleted in ICCs from diabetic mice. A conditional knockout of mir-10b in KIT+ cells or depletion of KIT+ cells in mice leads to degeneration of β cells and ICCs, resulting in diabetes and gastroparesis. miR-10b-5p targets the transcription factor Krüppel-like factor 11 (KLF11), which negatively regulates KIT expression. The miR-10b-5p mimic or Klf11 small interfering RNAs injected into mir-10b knockout mice, diet-induced diabetic mice, and TALLYHO polygenic diabetic mice rescue the diabetes and gastroparesis phenotype for an extended period of time. Furthermore, the miR-10b-5p mimic is more effective in improving glucose homoeostasis and gastrointestinal motility compared with common antidiabetic and prokinetic medications.
Conclusions: miR-10b-5p is a key regulator in diabetes and gastrointestinal dysmotility via the KLF11-KIT pathway. Restoration of miR-10b-5p may provide therapeutic benefits for these disorders.
Keywords: Diabetic Gastroparesis; Gastrointestinal Dysmotility; Interstitial Cells of Cajal; MicroRNAs; Pancreatic β Cells.
Copyright © 2021 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest statement (for all authors)
A provisional patent application entitled “miR-10b mimics and targets thereof for use in the treatment of diabetes and gastrointestinal motility disorders” has been filed by S.R. and the University of Nevada Reno Office of Technology Transfer (serial no. 62/837,988, filed April 24 2019).
Figures



References
-
- Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271–281. - PubMed
-
- Holman N, Young B, Gadsby R. Current prevalence of Type 1 and Type 2 diabetes in adults and children in the UK. Diabet Med 2015;32:1119–20. - PubMed
-
- Eizirik DL, Pasquali L, Cnop M. Pancreatic beta-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol 2020;16:349–362. - PubMed
-
- Cherney DZI, Lam TKT. A Gut Feeling for Metformin. Cell Metab 2018;28:808–810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

