Effect of Mitochondrial Antioxidant (Mito-TEMPO) on Burn-Induced Cardiac Dysfunction
- PMID: 33421567
- PMCID: PMC8753741
- DOI: 10.1016/j.jamcollsurg.2020.11.031
Effect of Mitochondrial Antioxidant (Mito-TEMPO) on Burn-Induced Cardiac Dysfunction
Abstract
Background: Imbalance of oxidants/antioxidants results in heart failure, contributing to mortality after burn injury. Cardiac mitochondria are a prime source of reactive oxygen species (ROS), and a mitochondrial-specific antioxidant may improve burn-induced cardiomyopathy. We hypothesize that the mitochondrial-specific antioxidant, Triphenylphosphonium chloride (Mito-TEMPO), could protect cardiac function after burn.
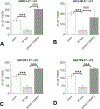
Study design: Male rats had a 60% total body surface area (TBSA) scald burn injury and were treated with/without Mito-TEMPO (7 mg/kg-1, intraperitoneal) and harvested at 24 hours post-burn. Echocardiography (ECHO) was used for measurement of heart function. Masson Trichrome and hematoxylin and eosin (H & E) staining were used for cardiac fibrosis and immune response. Qualitative polymerase chain reaction (qPCR) was used for mitochondrial DNA replication and gene expression.
Results: Burn-induced cardiac dysfunction, fibrosis, and mitochondrial damage were assessed by measurement of mitochondrial function, DNA replication, and DNA-encoded electron transport chain-related gene expression. Mito-TEMPO partially improved the abnormal parameters. Burn-induced cardiac dysfunction was associated with crosstalk between the NFE2L2-ARE pathway, PDE5A-PKG pathway, PARP1-POLG-mtDNA replication pathway, and mitochondrial SIRT signaling.
Conclusions: Mito-TEMPO reversed burn-induced cardiac dysfunction by rescuing cardiac mitochondrial dysfunction. Mitochondria-targeted antioxidants may be an effective therapy for burn-induced cardiac dysfunction.
Copyright © 2021 American College of Surgeons. Published by Elsevier Inc. All rights reserved.
Figures



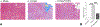



Comment in
-
Invited Commentary.J Am Coll Surg. 2021 Apr;232(4):655. doi: 10.1016/j.jamcollsurg.2020.12.029. J Am Coll Surg. 2021. PMID: 33771323 No abstract available.
References
-
- Miller SF, Bessey PQ, Schurr MJ, et al. National Burn Repository 2005: a ten-year review. J Burn Care Res. 2006. Jul-Aug;27(4):411–36. - PubMed
-
- Kallinen O, Maisniemi K, Bohling T, et al. Multiple organ failure as a cause of death in patients with severe burns. J Burn Care Res. 2012. Mar-Apr;33(2):206–11. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

