Nuclear envelope remodelling during mitosis
- PMID: 33421755
- PMCID: PMC8129912
- DOI: 10.1016/j.ceb.2020.12.004
Nuclear envelope remodelling during mitosis
Abstract
The defining feature of the eukaryotic cell, the nucleus, is bounded by a double envelope. This envelope and the nuclear pores within it play a critical role in separating the genome from the cytoplasm. It also presents cells with a challenge. How are cells to remodel the nuclear compartment boundary during mitosis without compromising nuclear function? In the two billion years since the emergence of the first cells with a nucleus, eukaryotes have evolved a range of strategies to do this. At one extreme, the nucleus is disassembled upon entry into mitosis and then reassembled anew in the two daughter cells. At the other, cells maintain an intact nuclear compartment boundary throughout the division process. In this review, we discuss common features of the division process that underpin remodelling mechanisms, the topological challenges involved and speculate on the selective pressures that may drive the evolution of distinct modes of division.
Keywords: Eukaryogenesis; Lamina; Mitosis; Nuclear division; Nuclear envelope; Nuclear pore complex.
Copyright © 2020 MRC Laboratory of Molecular Biology. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest statement Nothing declared.
Figures
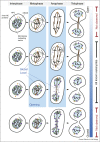
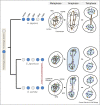
References
-
- De Magistris P., Antonin W. The dynamic nature of the nuclear envelope. Curr Biol. 2018;28:R487–R497. - PubMed
-
Excellent recent review of nuclear envelope dynamics, focussing on how cells solve the various topological challenges of mitosis as well as NPC insertion and assembly by remodelling the nuclear envelope.
-
- Hampoelz B., Andres-Pons A., Kastritis P., Beck M. Structure and assembly of the nuclear pore complex. Annu Rev Biophys. 2019;48:515–536. - PubMed
-
- Romanauska A., Köhler A. The inner nuclear membrane is a metabolically active territory that generates nuclear lipid droplets. Cell. 2018;174:700–715. e18. - PMC - PubMed
-
First demonstration of a unique metabolic activity traced to the inner nuclear envelope: enzymes directed to the inner nuclear envelope drive the synthesis of lipid droplets through lipid transfer regulated by Seipins.
-
- Smoyer C.J., Jaspersen S.L. Patrolling the nucleus: inner nuclear membrane-associated degradation. Curr Genet. 2019 doi: 10.1007/s00294-019-00971-1. - DOI - PMC - PubMed
-
Specific proteasomal activity clears misfolded and mis-localised proteins from the inner nuclear envelope and maintains the integrity of the nuclear proteome. A recent review focussed mostly on findings in yeast.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

