Development and evaluation of recombinant GRA8 protein for the serodiagnosis of Toxoplasma gondii infection in goats
- PMID: 33422085
- PMCID: PMC7796619
- DOI: 10.1186/s12917-020-02719-3
Development and evaluation of recombinant GRA8 protein for the serodiagnosis of Toxoplasma gondii infection in goats
Abstract
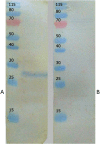
Background: The development of sensitive and specific methods for detecting Toxoplasma gondii infection is critical for preventing and controlling toxoplasmosis in humans and other animals. Recently, various recombinant proteins have been used in serological tests for diagnosing toxoplasmosis. The production of these antigens is associated with live tachyzoites obtained from cell cultures or laboratory animals for genomic extraction to amplify target genes. Synthetic genes have gained a key role in recombinant protein production. For the first time, we demonstrated the production of the recombinant protein of the T. gondii dense granular antigen 8 (TgGRA8) gene based on commercial gene synthesis. Recombinant TgGRA8 plasmids were successfully expressed in an Escherichia coli system. The recombinant protein was affinity-purified and characterized via sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting. Furthermore, the diagnostic potential of the recombinant protein was assessed using 306 field serum samples from goats via indirect enzyme-linked immunosorbent assay (iELISA) and the latex agglutination test (LAT).
Results: Western blotting using known positive serum samples from goats identified a single antigen at the expected molecular weight of TgGRA8 (27 kDa). iELISA illustrated that 15.40% of goat samples were positive for T. gondii-specific IgG antibodies. In addition, TgGRA8 provided high sensitivity and specificity, with significant concordance (91.83) and kappa values (0.69) compared with the results obtained using LAT.
Conclusion: Our findings highlight the production of a recombinant protein from a synthetic TgGRA8 gene and the ability to detect T. gondii infection in field samples. The sensitivity and specificity of TgGRA8 demonstrated that this protein could be a good serological marker for detecting specific IgG in goat sera.
Keywords: GRA8; Gene synthesis; Goat; Serodiagnosis; Toxoplasma gondii.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
The Development of Toxoplasma gondii Recombinant Trivalent Chimeric Proteins as an Alternative to Toxoplasma Lysate Antigen (TLA) in Enzyme-Linked Immunosorbent Assay (ELISA) for the Detection of Immunoglobulin G (IgG) in Small Ruminants.Int J Mol Sci. 2024 Apr 16;25(8):4384. doi: 10.3390/ijms25084384. Int J Mol Sci. 2024. PMID: 38673969 Free PMC article.
-
High-level expression of SAG1 and GRA7 gene of Toxoplasma gondii (Izatnagar isolate) and their application in serodiagnosis of goat toxoplasmosis.Vet Parasitol. 2008 Jul 4;154(3-4):185-92. doi: 10.1016/j.vetpar.2008.03.032. Epub 2008 Apr 16. Vet Parasitol. 2008. PMID: 18495348
-
Characterization and evaluation of a recombinant multiepitope peptide antigen MAG in the serological diagnosis of Toxoplasma gondii infection in pigs.Parasit Vectors. 2021 Aug 17;14(1):408. doi: 10.1186/s13071-021-04917-w. Parasit Vectors. 2021. PMID: 34404476 Free PMC article.
-
[Problems and limitations of conventional and innovative methods for the diagnosis of Toxoplasmosis in humans and animals].Parassitologia. 2004 Jun;46(1-2):177-81. Parassitologia. 2004. PMID: 15305712 Review. Italian.
-
Toxoplasma gondii recombinant antigens as tools for serodiagnosis of human toxoplasmosis: current status of studies.Clin Vaccine Immunol. 2013 Sep;20(9):1343-51. doi: 10.1128/CVI.00117-13. Epub 2013 Jun 19. Clin Vaccine Immunol. 2013. PMID: 23784855 Free PMC article. Review.
Cited by
-
Recombinant Dense Granule Protein (NcGRA4) Is a Novel Serological Marker for Neospora caninum Infection in Goats.Animals (Basel). 2023 Jun 5;13(11):1879. doi: 10.3390/ani13111879. Animals (Basel). 2023. PMID: 37889832 Free PMC article.
-
Development and evaluation of indirect enzyme-linked immunosorbent assay using recombinant dense granule antigen 7 protein for the detection of Toxoplasma gondii infection in cats in Thailand.Vet World. 2022 Mar;15(3):602-610. doi: 10.14202/vetworld.2022.602-610. Epub 2022 Mar 12. Vet World. 2022. PMID: 35497967 Free PMC article.
-
Detection of Toxoplasma gondii Infection in Small Ruminants: Old Problems, and Current Solutions.Animals (Basel). 2023 Aug 23;13(17):2696. doi: 10.3390/ani13172696. Animals (Basel). 2023. PMID: 37684960 Free PMC article. Review.
-
Mining the Proteome of Toxoplasma Parasites Seeking Vaccine and Diagnostic Candidates.Animals (Basel). 2022 Apr 23;12(9):1098. doi: 10.3390/ani12091098. Animals (Basel). 2022. PMID: 35565525 Free PMC article. Review.
-
The Development of Toxoplasma gondii Recombinant Trivalent Chimeric Proteins as an Alternative to Toxoplasma Lysate Antigen (TLA) in Enzyme-Linked Immunosorbent Assay (ELISA) for the Detection of Immunoglobulin G (IgG) in Small Ruminants.Int J Mol Sci. 2024 Apr 16;25(8):4384. doi: 10.3390/ijms25084384. Int J Mol Sci. 2024. PMID: 38673969 Free PMC article.
References
-
- Stelzera S, Bassob W, Benavides Silvánc J, Ortega-Morad LM, Maksimova P, Gethmanna J, Conrathsa FJ, Schare G. Toxoplasma gondii infection and toxoplasmosis in farm animals: risk factors and economic impact. Food Waterborne Parasitol. 2019;12:e00037. doi: 10.1016/j.fawpar.2019.e00037. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

