The EGFR-HSF1 axis accelerates the tumorigenesis of pancreatic cancer
- PMID: 33422093
- PMCID: PMC7797143
- DOI: 10.1186/s13046-020-01823-4
The EGFR-HSF1 axis accelerates the tumorigenesis of pancreatic cancer
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) is one of the most malignant diseases because of its non-symptomatic tumorigenesis. We previous found heat shock factor 1 (HSF1) was critical for PDAC progression and the aim of this study was to clarified the mechanisms on early activation of HSF1 and its role in the pancreatic cancer tumorigenesis.
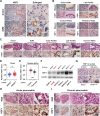
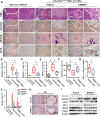
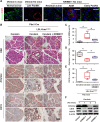
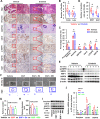
Methods: The expression and location of HSF1 on human or mice pancreatic tissues were examined by immunohistochemically staining. We mainly used pancreatic acinar cell 3-dimensional (3D) culture and a spontaneous pancreatic precancerous lesion mouse model called LSL-KrasG12D/+; Pdx1-Cre (KC) (and pancreatitis models derived from KC mice) to explore the pro-tumorigenesis mechanisms of the HSF1 in vitro and in vivo. Bioinformatics and molecular experiments were used to explore the underlying mechanisms between HSF1 and epidermal growth factor receptor (EGFR).
Results: In this study, we found that pharmacological inhibition of HSF1 slowed pancreatic cancer initiation and suppressed the pancreatitis-induced formation of pancreatic precancerous lesion. Next, bioinformatics analysis revealed the closely linked between HSF1 and EGFR pathway and we also confirmed their parallel activation in pancreatic precancerous lesions. Besides, the pharmacological inhibition of EGFR suppressed the initiation of pancreatic cancer and the activation of HSF1 in vivo. Indeed, we demonstrated that the EGFR activation that mediated pancreatic cancer tumorigenesis was partly HSF1-dependent in vitro.
Conclusion: Hence, we concluded that the EGFR-HSF1 axis promoted the initiation of pancreatic cancer.
Keywords: Epidermal growth factor receptor; Heat shock factor 1; Pancreatic ductal adenocarcinoma; Transgenic mice; Tumorigenesis.
Conflict of interest statement
The authors declare that they have no competing financial and non-financial interest.
Figures


References
-
- Awasthi N, Kronenberger D, Stefaniak A, Hassan MS, von Holzen U, Schwarz MA, Schwarz RE. Dual inhibition of the PI3K and MAPK pathways enhances nab-paclitaxel/gemcitabine chemotherapy response in preclinical models of pancreatic cancer. Cancer Lett. 2019;459:41–49. doi: 10.1016/j.canlet.2019.05.037. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

