The impact of dextran sodium sulphate and probiotic pre-treatment in a murine model of Parkinson's disease
- PMID: 33422110
- PMCID: PMC7796536
- DOI: 10.1186/s12974-020-02062-2
The impact of dextran sodium sulphate and probiotic pre-treatment in a murine model of Parkinson's disease
Abstract
Background: Recent work has established that Parkinson's disease (PD) patients have an altered gut microbiome, along with signs of intestinal inflammation. This could help explain the high degree of gastric disturbances in PD patients, as well as potentially be linked to the migration of peripheral inflammatory factors into the brain. To our knowledge, this is the first study to examine microbiome alteration prior to the induction of a PD murine model.
Methods: We presently assessed whether pre-treatment with the probiotic, VSL #3, or the inflammatory inducer, dextran sodium sulphate (DSS), would influence the PD-like pathology provoked by a dual hit toxin model using lipopolysaccharide (LPS) and paraquat exposure.
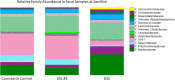
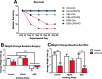
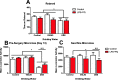
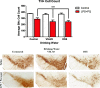
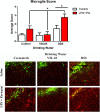
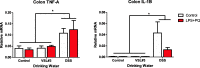
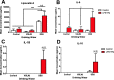
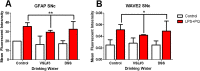
Results: While VSL #3 has been reported to have anti-inflammatory effects, DSS is often used as a model of colitis because of the gut inflammation and the breach of the intestinal barrier that it induces. We found that VSL#3 did not have any significant effects (beyond a blunting of LPS paraquat-induced weight loss). However, the DSS treatment caused marked changes in the gut microbiome and was also associated with augmented behavioral and inflammatory outcomes. In fact, DSS markedly increased taxa belonging to the Bacteroidaceae and Porphyromonadaceae families but reduced those from Rikencellaceae and S24-7, as well as provoking colonic pro-inflammatory cytokine expression, consistent with an inflamed gut. The DSS also increased the impact of LPS plus paraquat upon microglial morphology, along with circulating lipocalin-2 (neutrophil marker) and IL-6. Yet, neither DSS nor VSL#3 influenced the loss of substantia nigra dopamine neurons or the astrocytic and cytoskeleton remodeling protein changes that were provoked by the LPS followed by paraquat treatment.
Conclusions: These data suggest that disruption of the intestinal integrity and the associated microbiome can interact with systemic inflammatory events to promote widespread brain-gut changes that could be relevant for PD and at the very least, suggestive of novel neuro-immune communication.
Keywords: Inflammatory neurodege5neration; Microbiota; Microglia; Probiotic.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Intestinal anti-inflammatory effect of the probiotic Saccharomyces boulardii in DSS-induced colitis in mice: Impact on microRNAs expression and gut microbiota composition.J Nutr Biochem. 2018 Nov;61:129-139. doi: 10.1016/j.jnutbio.2018.08.005. Epub 2018 Sep 1. J Nutr Biochem. 2018. PMID: 30236870
-
Inflammatory priming of the substantia nigra influences the impact of later paraquat exposure: Neuroimmune sensitization of neurodegeneration.Neurobiol Aging. 2009 Sep;30(9):1361-78. doi: 10.1016/j.neurobiolaging.2007.11.020. Epub 2008 Jan 10. Neurobiol Aging. 2009. PMID: 18187236
-
Modulation of Intestinal Inflammation and Protection of Dopaminergic Neurons in Parkinson's Disease Mice through a Probiotic Formulation Targeting NLRP3 Inflammasome.J Neuroimmune Pharmacol. 2025 Jan 18;20(1):9. doi: 10.1007/s11481-024-10163-5. J Neuroimmune Pharmacol. 2025. PMID: 39826038 Free PMC article.
-
Poloxamer 188-mediated anti-inflammatory effect rescues cognitive deficits in paraquat and maneb-induced mouse model of Parkinson's disease.Toxicology. 2020 Apr 30;436:152437. doi: 10.1016/j.tox.2020.152437. Epub 2020 Mar 10. Toxicology. 2020. PMID: 32169474
-
Chronic stress-induced gut dysfunction exacerbates Parkinson's disease phenotype and pathology in a rotenone-induced mouse model of Parkinson's disease.Neurobiol Dis. 2020 Feb;135:104352. doi: 10.1016/j.nbd.2018.12.012. Epub 2018 Dec 21. Neurobiol Dis. 2020. PMID: 30579705 Review.
Cited by
-
Impact of aging on animal models of Parkinson's disease.Front Aging Neurosci. 2022 Jul 28;14:909273. doi: 10.3389/fnagi.2022.909273. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35966779 Free PMC article.
-
Intestinal barrier permeability: the influence of gut microbiota, nutrition, and exercise.Front Physiol. 2024 Jul 8;15:1380713. doi: 10.3389/fphys.2024.1380713. eCollection 2024. Front Physiol. 2024. PMID: 39040079 Free PMC article. Review.
-
Systematic review and meta-analysis of microbiota-gut-astrocyte axis perturbation in neurodegeneration, brain injury, and mood disorders.Brain Behav Immun Health. 2025 May 12;46:101013. doi: 10.1016/j.bbih.2025.101013. eCollection 2025 Jul. Brain Behav Immun Health. 2025. PMID: 40485663 Free PMC article. Review.
-
The Gut-Brain Axis and Its Relation to Parkinson's Disease: A Review.Front Aging Neurosci. 2022 Jan 7;13:782082. doi: 10.3389/fnagi.2021.782082. eCollection 2021. Front Aging Neurosci. 2022. PMID: 35069178 Free PMC article. Review.
-
Intestinal Barrier Dysfunction in the Absence of Systemic Inflammation Fails to Exacerbate Motor Dysfunction and Brain Pathology in a Mouse Model of Parkinson's Disease.Front Neurol. 2022 May 18;13:882628. doi: 10.3389/fneur.2022.882628. eCollection 2022. Front Neurol. 2022. PMID: 35665034 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

