Structure, kinetic properties and biological function of mechanosensitive Piezo channels
- PMID: 33422128
- PMCID: PMC7796548
- DOI: 10.1186/s13578-020-00522-z
Structure, kinetic properties and biological function of mechanosensitive Piezo channels
Abstract
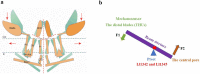
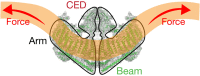
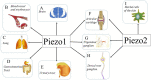
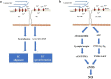
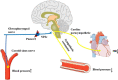
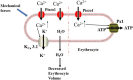
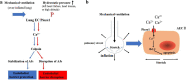
Mechanotransduction couples mechanical stimulation with ion flux, which is critical for normal biological processes involved in neuronal cell development, pain sensation, and red blood cell volume regulation. Although they are key mechanotransducers, mechanosensitive ion channels in mammals have remained difficult to identify. In 2010, Coste and colleagues revealed a novel family of mechanically activated cation channels in eukaryotes, consisting of Piezo1 and Piezo2 channels. These have been proposed as the long-sought-after mechanosensitive cation channels in mammals. Piezo1 and Piezo2 exhibit a unique propeller-shaped architecture and have been implicated in mechanotransduction in various critical processes, including touch sensation, balance, and cardiovascular regulation. Furthermore, several mutations in Piezo channels have been shown to cause multiple hereditary human disorders, such as autosomal recessive congenital lymphatic dysplasia. Notably, mutations that cause dehydrated hereditary xerocytosis alter the rate of Piezo channel inactivation, indicating the critical role of their kinetics in normal physiology. Given the importance of Piezo channels in understanding the mechanotransduction process, this review focuses on their structural details, kinetic properties and potential function as mechanosensors. We also briefly review the hereditary diseases caused by mutations in Piezo genes, which is key for understanding the function of these proteins.
Keywords: Function; Ion channel; Mechanotransduction; Piezo.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

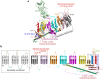


Similar articles
-
In Touch With the Mechanosensitive Piezo Channels: Structure, Ion Permeation, and Mechanotransduction.Curr Top Membr. 2017;79:159-195. doi: 10.1016/bs.ctm.2016.11.006. Epub 2017 Feb 21. Curr Top Membr. 2017. PMID: 28728816 Review.
-
Genetic Diseases of PIEZO1 and PIEZO2 Dysfunction.Curr Top Membr. 2017;79:97-134. doi: 10.1016/bs.ctm.2017.01.001. Epub 2017 Feb 28. Curr Top Membr. 2017. PMID: 28728825 Review.
-
A Tour de Force: The Discovery, Properties, and Function of Piezo Channels.Curr Top Membr. 2017;79:1-36. doi: 10.1016/bs.ctm.2016.11.007. Epub 2017 Jan 11. Curr Top Membr. 2017. PMID: 28728814 Review.
-
The mechanosensitive Piezo1 channel: structural features and molecular bases underlying its ion permeation and mechanotransduction.J Physiol. 2018 Mar 15;596(6):969-978. doi: 10.1113/JP274404. Epub 2017 Dec 19. J Physiol. 2018. PMID: 29171028 Free PMC article. Review.
-
Structural Designs and Mechanogating Mechanisms of the Mechanosensitive Piezo Channels.Trends Biochem Sci. 2021 Jun;46(6):472-488. doi: 10.1016/j.tibs.2021.01.008. Epub 2021 Feb 17. Trends Biochem Sci. 2021. PMID: 33610426 Review.
Cited by
-
Mechanosignals in abdominal aortic aneurysms.Front Cardiovasc Med. 2023 Jan 9;9:1021934. doi: 10.3389/fcvm.2022.1021934. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36698932 Free PMC article. Review.
-
Mechanotransductive receptor Piezo1 as a promising target in the treatment of fibrosis diseases.Front Mol Biosci. 2023 Oct 12;10:1270979. doi: 10.3389/fmolb.2023.1270979. eCollection 2023. Front Mol Biosci. 2023. PMID: 37900917 Free PMC article. Review.
-
Prolonged Piezo1 Activation Induces Cardiac Arrhythmia.Int J Mol Sci. 2023 Apr 4;24(7):6720. doi: 10.3390/ijms24076720. Int J Mol Sci. 2023. PMID: 37047693 Free PMC article.
-
Anatomy and transcriptomics of the common jingle shell (Bivalvia, Anomiidae) support a sensory function for bivalve tentacles.Sci Rep. 2024 Dec 28;14(1):31539. doi: 10.1038/s41598-024-83313-7. Sci Rep. 2024. PMID: 39733126 Free PMC article.
-
Piezo channels in the intestinal tract.Front Physiol. 2024 Feb 6;15:1356317. doi: 10.3389/fphys.2024.1356317. eCollection 2024. Front Physiol. 2024. PMID: 38379701 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

