Intravitreal autologous mesenchymal stem cell transplantation: a non-randomized phase I clinical trial in patients with retinitis pigmentosa
- PMID: 33422139
- PMCID: PMC7796606
- DOI: 10.1186/s13287-020-02122-7
Intravitreal autologous mesenchymal stem cell transplantation: a non-randomized phase I clinical trial in patients with retinitis pigmentosa
Abstract
Background: Retinitis pigmentosa (RP) is a progressive inherited retinal disease with great interest for finding effective treatment modalities. Stem cell-based therapy is one of the promising candidates. We aimed to investigate the safety, feasibility, and short-term efficacy of intravitreal injection of bone marrow-derived mesenchymal stem cells (BM-MSCs) in participants with advanced stage RP.
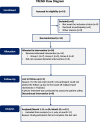
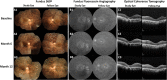
Methods: This non-randomized phase I clinical trial enrolled 14 participants, categorized into three groups based on a single dose intravitreal BM-MSC injection of 1 × 106, 5 × 106, or 1 × 107 cells. We evaluated signs of inflammation and other adverse events (AEs). We also assessed the best corrected visual acuity (BCVA), visual field (VF), central subfield thickness (CST), and subjective experiences.
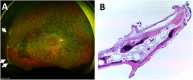
Results: During the 12-month period, we noticed several mild and transient AEs. Interestingly, we found statistically significant improvements in the BCVA compared to baseline, although they returned to the baseline at 12 months. The VF and CST were stable, indicating no remarkable disease progression. We followed 12 participants beyond the study period, ranging from 1.5 to 7 years, and observed one severe but manageable AE at year 3.
Conclusion: Intravitreal injection of BM-MSCs appears to be safe and potentially effective. All adverse events during the 12-month period required observation without any intervention. For the long-term follow-up, only one participant needed surgical treatment for a serious adverse event and the vision was restored. An enrollment of larger number of participants with less advanced RP and long-term follow-up is required to evaluate the safety and efficacy of this intervention.
Trial registration: ClinicalTrials.gov, NCT01531348 . Registered on February 10, 2012.
Keywords: Inherited retinal diseases; Mesenchymal stem cell; Phase I clinical trial; Retinitis pigmentosa (RP); Stem cell therapy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Management of retinitis pigmentosa by Wharton's jelly-derived mesenchymal stem cells: prospective analysis of 1-year results.Stem Cell Res Ther. 2020 Aug 12;11(1):353. doi: 10.1186/s13287-020-01870-w. Stem Cell Res Ther. 2020. PMID: 32787913 Free PMC article. Clinical Trial.
-
Subretinal adipose tissue-derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: a phase I clinical safety study.Stem Cell Res Ther. 2016 Dec 1;7(1):178. doi: 10.1186/s13287-016-0432-y. Stem Cell Res Ther. 2016. PMID: 27906070 Free PMC article. Clinical Trial.
-
Management of retinitis pigmentosa by Wharton's jelly derived mesenchymal stem cells: preliminary clinical results.Stem Cell Res Ther. 2020 Jan 13;11(1):25. doi: 10.1186/s13287-020-1549-6. Stem Cell Res Ther. 2020. PMID: 31931872 Free PMC article.
-
Efficacy and safety of mesenchymal stem cell therapies in retinitis pigmentosa: a systematic review and meta-analysis.Int Ophthalmol. 2025 Mar 12;45(1):85. doi: 10.1007/s10792-025-03478-6. Int Ophthalmol. 2025. PMID: 40072800
-
Recent advances of stem cell therapy for retinitis pigmentosa.Int J Mol Sci. 2014 Aug 20;15(8):14456-74. doi: 10.3390/ijms150814456. Int J Mol Sci. 2014. PMID: 25141102 Free PMC article. Review.
Cited by
-
Retinitis Pigmentosa: From Pathomolecular Mechanisms to Therapeutic Strategies.Medicina (Kaunas). 2024 Jan 22;60(1):189. doi: 10.3390/medicina60010189. Medicina (Kaunas). 2024. PMID: 38276069 Free PMC article. Review.
-
Stem Cell-Based Therapeutic Approaches in Genetic Diseases.Adv Exp Med Biol. 2023;1436:19-53. doi: 10.1007/5584_2023_761. Adv Exp Med Biol. 2023. PMID: 36735185
-
Stem Cell Therapy for Retinal Degeneration: The Evidence to Date.Biologics. 2021 Jul 27;15:299-306. doi: 10.2147/BTT.S290331. eCollection 2021. Biologics. 2021. PMID: 34349498 Free PMC article. Review.
-
Retinitis Pigmentosa: Current Clinical Management and Emerging Therapies.Int J Mol Sci. 2023 Apr 19;24(8):7481. doi: 10.3390/ijms24087481. Int J Mol Sci. 2023. PMID: 37108642 Free PMC article. Review.
-
Gene-agnostic approaches to treating inherited retinal degenerations.Front Cell Dev Biol. 2023 Apr 13;11:1177838. doi: 10.3389/fcell.2023.1177838. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37123404 Free PMC article. Review.
References
-
- RetNet, the Retinal Information Network (2020).
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

