Metabolic phenotyping reveals a reduction in the bioavailability of serotonin and kynurenine pathway metabolites in both the urine and serum of individuals living with Alzheimer's disease
- PMID: 33422142
- PMCID: PMC7797094
- DOI: 10.1186/s13195-020-00741-z
Metabolic phenotyping reveals a reduction in the bioavailability of serotonin and kynurenine pathway metabolites in both the urine and serum of individuals living with Alzheimer's disease
Abstract
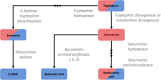
Background: Both serotonergic signalling disruption and systemic inflammation have been associated with the pathogenesis of Alzheimer's disease (AD). The common denominator linking the two is the catabolism of the essential amino acid, tryptophan. Metabolism via tryptophan hydroxylase results in serotonin synthesis, whilst metabolism via indoleamine 2,3-dioxygenase (IDO) results in kynurenine and its downstream derivatives. IDO is reported to be activated in times of host systemic inflammation and therefore is thought to influence both pathways. To investigate metabolic alterations in AD, a large-scale metabolic phenotyping study was conducted on both urine and serum samples collected from a multi-centre clinical cohort, consisting of individuals clinically diagnosed with AD, mild cognitive impairment (MCI) and age-matched controls.
Methods: Metabolic phenotyping was applied to both urine (n = 560) and serum (n = 354) from the European-wide AddNeuroMed/Dementia Case Register (DCR) biobank repositories. Metabolite data were subsequently interrogated for inter-group differences; influence of gender and age; comparisons between two subgroups of MCI - versus those who remained cognitively stable at follow-up visits (sMCI); and those who underwent further cognitive decline (cMCI); and the impact of selective serotonin reuptake inhibitor (SSRI) medication on metabolite concentrations.
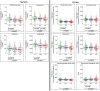
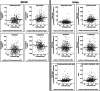
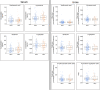
Results: Results revealed significantly lower metabolite concentrations of tryptophan pathway metabolites in the AD group: serotonin (urine, serum), 5-hydroxyindoleacetic acid (urine), kynurenine (serum), kynurenic acid (urine), tryptophan (urine, serum), xanthurenic acid (urine, serum), and kynurenine/tryptophan ratio (urine). For each listed metabolite, a decreasing trend in concentrations was observed in-line with clinical diagnosis: control > MCI > AD. There were no significant differences in the two MCI subgroups whilst SSRI medication status influenced observations in serum, but not urine.
Conclusions: Urine and serum serotonin concentrations were found to be significantly lower in AD compared with controls, suggesting the bioavailability of the neurotransmitter may be altered in the disease. A significant increase in the kynurenine/tryptophan ratio suggests that this may be a result of a shift to the kynurenine metabolic route due to increased IDO activity, potentially as a result of systemic inflammation. Modulation of the pathways could help improve serotonin bioavailability and signalling in AD patients.
Keywords: Alzheimer’s disease; Kynurenine; Mass spectrometry; Metabolic phenotyping; Serotonergic signalling; Serotonin; Systemic inflammation; Tryptophan.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

