Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial
- PMID: 33422263
- PMCID: PMC7832152
- DOI: 10.1016/S2213-2600(20)30558-0
Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial
Abstract
Background: Biological considerations suggest that renin-angiotensin system inhibitors might influence the severity of COVID-19. We aimed to evaluate whether continuing versus discontinuing renin-angiotensin system inhibitors (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) affects outcomes in patients admitted to hospital with COVID-19.
Methods: The REPLACE COVID trial was a prospective, randomised, open-label trial done at 20 large referral hospitals in seven countries worldwide. Eligible participants were aged 18 years and older who were admitted to hospital with COVID-19 and were receiving a renin-angiotensin system inhibitor before admission. Individuals with contraindications to continuation or discontinuation of renin-angiotensin system inhibitor therapy were excluded. Participants were randomly assigned (1:1) to continuation or discontinuation of their renin-angiotensin system inhibitor using permuted block randomisation, with allocation concealed using a secure web-based randomisation system. The primary outcome was a global rank score in which participants were ranked across four hierarchical tiers incorporating time to death, duration of mechanical ventilation, time on renal replacement or vasopressor therapy, and multiorgan dysfunction during the hospitalisation. Primary analyses were done in the intention-to-treat population. The REPLACE COVID trial is registered with ClinicalTrials.gov, NCT04338009.
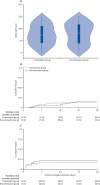
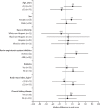
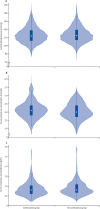
Findings: Between March 31 and Aug 20, 2020, 152 participants were enrolled and randomly assigned to either continue or discontinue renin-angiotensin system inhibitor therapy (continuation group n=75; discontinuation group n=77). Mean age of participants was 62 years (SD 12), 68 (45%) were female, mean body-mass index was 33 kg/m2 (SD 8), and 79 (52%) had diabetes. Compared with discontinuation of renin-angiotensin system inhibitors, continuation had no effect on the global rank score (median rank 73 [IQR 40-110] for continuation vs 81 [38-117] for discontinuation; β-coefficient 8 [95% CI -13 to 29]). There were 16 (21%) of 75 participants in the continuation arm versus 14 (18%) of 77 in the discontinuation arm who required intensive care unit admission or invasive mechanical ventilation, and 11 (15%) of 75 participants in the continuation group versus ten (13%) of 77 in the discontinuation group died. 29 (39%) participants in the continuation group and 28 (36%) participants in the discontinuation group had at least one adverse event (χ2 test of adverse events between treatment groups p=0·77). There was no difference in blood pressure, serum potassium, or creatinine during follow-up across the two groups.
Interpretation: Consistent with international society recommendations, renin-angiotensin system inhibitors can be safely continued in patients admitted to hospital with COVID-19.
Funding: REPLACE COVID Investigators, REPLACE COVID Trial Social Fundraising Campaign, and FastGrants.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Renin-angiotensin system inhibitors in hospitalised patients with COVID-19.Lancet Respir Med. 2021 Mar;9(3):221-222. doi: 10.1016/S2213-2600(21)00003-5. Epub 2021 Jan 7. Lancet Respir Med. 2021. PMID: 33422264 Free PMC article. No abstract available.
-
From confusion to clarity: RAS blockade in patients hospitalized with COVID-19.Kidney Int. 2021 May;99(5):1059-1061. doi: 10.1016/j.kint.2021.02.034. Epub 2021 Mar 19. Kidney Int. 2021. PMID: 33753072 Free PMC article. No abstract available.
References
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- K23 HL133843/HL/NHLBI NIH HHS/United States
- P30 DK092926/DK/NIDDK NIH HHS/United States
- R03 HL146874/HL/NHLBI NIH HHS/United States
- R01 HL104106/HL/NHLBI NIH HHS/United States
- R61 HL146390/HL/NHLBI NIH HHS/United States
- R01 HL121510/HL/NHLBI NIH HHS/United States
- R56 HL136730/HL/NHLBI NIH HHS/United States
- R01 AG058969/AG/NIA NIH HHS/United States
- P01 HL094307/HL/NHLBI NIH HHS/United States
- T32 DK007785/DK/NIDDK NIH HHS/United States
- T32 HL007891/HL/NHLBI NIH HHS/United States
- R01 HL153646/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

