Biomarker-Based Risk Prediction of Incident Heart Failure in Pre-Diabetes and Diabetes
- PMID: 33422434
- PMCID: PMC11229674
- DOI: 10.1016/j.jchf.2020.10.013
Biomarker-Based Risk Prediction of Incident Heart Failure in Pre-Diabetes and Diabetes
Abstract
Objectives: This study evaluated the application of a biomarker-based risk score to identify individuals with dysglycemia who are at high risk for incident heart failure (HF) and to inform allocation of effective preventive interventions.
Background: Risk stratification tools to identify patients with diabetes and pre-diabetes at highest risk for HF are needed to inform cost-effective allocation of preventive therapies. Whether a biomarker score can meaningfully stratify HF risk is unknown.
Methods: Participants free of cardiovascular disease from 3 cohort studies (ARIC [Atherosclerosis Risk In Communities], DHS [Dallas Heart Study], and MESA [Multi-Ethnic Study of Atherosclerosis]) were included. An integer-based biomarker score included high-sensitivity cardiac troponin T ≥6 ng/l, N-terminal pro-B-type natriuretic peptide ≥125 pg/ml, high-sensitivity C-reactive protein ≥3 mg/l, and left ventricular hypertrophy by electrocardiography, with 1 point for each abnormal parameter. The 5-year risk of HF was estimated among participants with diabetes and pre-diabetes across biomarker score groups (0 to 4).
Results: The primary analysis included 6,799 participants with dysglycemia (diabetes: 33.2%; pre-diabetes: 66.8%). The biomarker score demonstrated good discrimination and calibration for predicting 5- and 10-year HF risk among pre-diabetes and diabetes cohorts. The 5-year risk of HF among subjects with a biomarker score of ≤1 was low and comparable to participants with euglycemia (0.78%). The 5-year risk for HF increased in a graded fashion with an increasing biomarker score, with the highest risk noted among those with scores of ≥3 (diabetes: 12.0%; pre-diabetes: 7.8%). The estimated number of HF events that could be prevented using a sodium-glucose cotransporter-2 inhibitor per 1,000 treated subjects over 5 years was 11 for all subjects with diabetes and ranged from 4 in the biomarker score zero group to 44 in the biomarker score ≥3 group.
Conclusions: Among adults with diabetes and pre-diabetes, a biomarker score can stratify HF risk and inform allocation of HF prevention therapies.
Keywords: SGLT-2 inhibitors; biomarkers; diabetes; pre-diabetes; risk prediction.
Copyright © 2021 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures Dr. Pandey was supported by the Texas Health Resources Clinical Scholarship, Gilead Sciences Research Scholar Program, and the National Institute of Aging GEMSSTAR Grant (1R03AG067960-01). The ARIC study is conducted as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN2 68201100006C, HHSN268201100007C, HHSN268201100008C, HHSN26820 1100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201 100012C). The Dallas Heart Study was funded by a grant from the Donald W. Reynolds Foundation. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (award number UL1TR001105) to the University of Texas Southwestern Medical Center. The Multi-Ethnic Study of Atherosclerosis was supported by the National Heart, Lung, and Blood Institute (R01 HL071739 and contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, and N01-C-95169). Dr. Vaduganathan has been supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541); and has served on advisory boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Cytokinetics, and Relypsa. Dr. Pandey has served on the advisory board of Roche Diagnostics. Dr. Patel was supported by the National Heart, Lung, and Blood Institute T32 postdoctoral training grant (5T32HL125247-03). Dr. Kosiborod has received research grant support from AstraZeneca and Boehringer Ingelheim; has served as a consultant or on advisory boards for Amgen, Applied Therapeutics, Astra Zeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck (Diabetes), Novo Nordisk, Sanofi, and Vifor Pharma; has received other research support from AstraZeneca and honorarium from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk. Dr. de Lemos has received grant support from Roche Diagnostics and Abbott Diagnostics; and has received consulting fees from Roche Diagnostics, Abbott Diagnostics, Ortho Clinical Diagnostics, Siemen’s Health Care Diagnostics, Quidel Cardiovascular, Inc, Novo Nordisk, Amgen, Regeneron, Eli Lilly, and Esperion. Drs. DeFilippi and de Lemos hold a patent pending (U.S. Patent Number: 15/309,754) entitled: “Methods for Assessing Differential Risk for Developing Heart Failure.” Dr. McGuire has received personal fees for trial leadership from GlaxoSmithKline, Janssen, Lexicon, AstraZeneca, CSL Behring, Sanofi, Boehringer Ingelheim, Merck & Co, Pfizer, Novo Nordisk, Eisai Inc., Esperion, and Lilly USA; and has received personal consultancy fees from AstraZeneca, Lilly USA, Boehringer Ingelheim, Merck & Co, Novo Nordisk, Metavant, Applied Therapeutics, Sanofi, and Afimmune. Dr. Everett has received personal consultancy fees from Amarin, Amgen, Gilead, FDA, Merck & Co., National Institute of Diabetes and Digestive and Kidney Diseases, and Roche Diagnostics outside the present work; and has received significant investigator-initiated grant funding from the National Institute of Heart, Lung, and Blood Institute outside the present work. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Figures
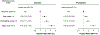


References
-
- National Diabetes Statistics Report. https://www.cdc.gov/diabetes/data/statistics/statistics-report.html. Accessed May 13, 2020.
-
- Khan H, Anker SD, Januzzi JL Jr. et al. Heart Failure Epidemiology in Patients With Diabetes Mellitus Without Coronary Heart Disease. J Card Fail 2019;25:78–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN268201100012C/HL/NHLBI NIH HHS/United States
- N01 HC095161/HL/NHLBI NIH HHS/United States
- HHSN268201100010C/HL/NHLBI NIH HHS/United States
- HHSN268201100007C/HL/NHLBI NIH HHS/United States
- HHSN268201100011C/HL/NHLBI NIH HHS/United States
- N01 HC095163/HL/NHLBI NIH HHS/United States
- HHSN268201100007I/HL/NHLBI NIH HHS/United States
- HHSN268201100006C/HL/NHLBI NIH HHS/United States
- N01 HC095162/HL/NHLBI NIH HHS/United States
- N01 HC095160/HL/NHLBI NIH HHS/United States
- HHSN268201100009I/HL/NHLBI NIH HHS/United States
- R01 HL071739/HL/NHLBI NIH HHS/United States
- UL1 TR001105/TR/NCATS NIH HHS/United States
- HHSN268201100008C/HL/NHLBI NIH HHS/United States
- N01 HC095169/HL/NHLBI NIH HHS/United States
- HHSN268201100005G/HL/NHLBI NIH HHS/United States
- HHSN268201100008I/HL/NHLBI NIH HHS/United States
- R03 AG067960/AG/NIA NIH HHS/United States
- HHSN268201100011I/HL/NHLBI NIH HHS/United States
- T32 HL125247/HL/NHLBI NIH HHS/United States
- N01 HC095159/HL/NHLBI NIH HHS/United States
- HHSN268201100005I/HL/NHLBI NIH HHS/United States
- UL1 TR002541/TR/NCATS NIH HHS/United States
- HHSN268201100009C/HL/NHLBI NIH HHS/United States
- HHSN268201100005C/HL/NHLBI NIH HHS/United States
- N01 HC095165/HL/NHLBI NIH HHS/United States
- N01 HC095164/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

