Remdesivir for the treatment of COVID-19: A systematic review and meta-analysis of randomized controlled trials
- PMID: 33422642
- PMCID: PMC7789823
- DOI: 10.1016/j.cct.2021.106272
Remdesivir for the treatment of COVID-19: A systematic review and meta-analysis of randomized controlled trials
Abstract
Background: The nucleotide analogue prodrug remdesivir was among the first antiviral therapies to be tested in randomized controlled trials (RCTs) for COVID-19. We performed a meta-analysis to understand efficacy and safety.
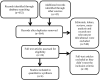
Methods: We searched PubMed, EMBASE, Cochrane library, and ClinicalTrials.gov databases (from January 1, 2020 to November 5, 2020). We included RCTs comparing the efficacy and safety of remdesivir to control/placebo in COVID-19. Two independent investigators abstracted data, assessed the quality of evidence, and rated the certainty of evidence.
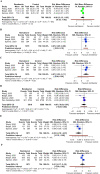
Results: A total of 4 RCTs with 7334 patients with COVID-19 were included. At a follow-up of 28-29 days from randomization, very low certainty evidence showed that use of remdesivir compared with control group (placebo and/or standard of care) was not associated with a significant decrease in time to clinical improvement (standardized mean difference -0.80 day; [CI, -2.12, 0.53]). However, moderate certainty of evidence showed that remdesivir was associated with higher rates of recovered patients (risk difference [RD] 0.07 [0.05, 0.08]) and discharged patients (RD 0.07 [0.03, 0.11]) and lower rates of developing serious adverse events (RD -0.05 [-0.10, -0.01]) compared with control. Moderate and very low certainty of evidence showed there was no significant difference in deaths at 28-29 days follow-up (RD -0.01 [-0.03, 0.01]) and developing any adverse events (RD 0.01 [-0.17, 0.19]) between both groups, respectively.
Conclusion: Patients given remdesivir are more likely to demonstrate recovery and were associated with higher rates of hospital discharge, but not with significant reduction in mean time to clinical improvement or mortality.
Keywords: COVID-19; Remdesivir; SARS-Cov-2.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous