Endothelial cells and SARS-CoV-2: An intimate relationship
- PMID: 33422689
- PMCID: PMC7834309
- DOI: 10.1016/j.vph.2021.106829
Endothelial cells and SARS-CoV-2: An intimate relationship
Abstract
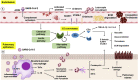
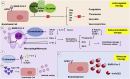
Angiotensin-converting enzyme 2 (ACE2) is an important player of the renin-angiotensin-aldosterone system (RAAS) in regulating the conversion of angiotensin II into angiotensin (1-7). While expressed on the surface of human cells, such as lung, heart, kidney, neurons, and endothelial cells (EC), ACE2 is the entry receptor for SARS-CoV-2. Here, we would like to highlight that ACE2 is predominant on the EC membrane. Many of coronavirus disease 2019 (COVID-19) symptoms have been associated with the large recruitment of immune cells, directly affecting EC. Additionally, cytokines, hypoxia, and complement activation can trigger the activation of EC leading to the coagulation cascade. The EC dysfunction plus the inflammation due to SARS-CoV-2 infection may lead to abnormal coagulation, actively participating in thrombo-inflammatory processes resulting in vasculopathy and indicating poor prognosis in patients with COVID-19. Considering the intrinsic relationship between EC and the pathophysiology of SARS-CoV-2, EC-associated therapies such as anticoagulants, fibrinolytic drugs, immunomodulators, and molecular therapies have been proposed. In this review, we will discuss the role of EC in the lung inflammation and edema, in the disseminate coagulation process, ACE2 positive cancer patients, and current and future EC-associated therapies to treat COVID-19.
Keywords: ACE2; Coagulation; Endothelial cells; Inflammation; SARS-CoV-2.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
None.
Figures


References
-
- Goshua G., Pine A.B., Meizlish M.L., Chang C.-H., Zhang H., Bahel P., Baluha A., Bar N., Bona R.D., Burns A.J., Dela Cruz C.S., Dumont A., Halene S., Hwa J., Koff J., Menninger H., Neparidze N., Price C., Siner J.M., Tormey C., Rinder H.M., Chun H.J., Lee A.I. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

