The EMA assessment of encorafenib in combination with cetuximab for the treatment of adult patients with metastatic colorectal carcinoma harbouring the BRAFV600E mutation who have received prior therapy
- PMID: 33422765
- PMCID: PMC7809377
- DOI: 10.1016/j.esmoop.2020.100031
The EMA assessment of encorafenib in combination with cetuximab for the treatment of adult patients with metastatic colorectal carcinoma harbouring the BRAFV600E mutation who have received prior therapy
Abstract
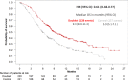
On 2 June 2020, a marketing authorisation valid through the European Union (EU) was issued for encorafenib in combination with cetuximab in adult patients with metastatic colorectal carcinoma (mCRC) with the BRAFV600E mutation who had received prior systemic therapy. Encorafenib plus cetuximab was evaluated in a randomised phase III trial of encorafenib plus binimetinib plus cetuximab versus encorafenib plus cetuximab versus cetuximab plus irinotecan or FOLFIRI (control arm) to adult patients with BRAFV600E mCRC who had received prior therapy for metastatic disease. The median overall survival was 9.3 months [95% confidence interval (CI): 8.05-11.30] versus 5.88 months (95% CI: 5.09-7.10) for encorafenib plus cetuximab (doublet) versus the control arm, respectively [hazard ratio (HR) 0.61, 95% CI: 0.48-0.77]. Progression-free survival (PFS) was 4.27 months (95% CI: 4.07-5.45) versus 1.54 months (95% CI: 1.48-1.91) (HR 0.44; 95% CI: 0.35-0.55). The most frequent adverse events in patients receiving encorafenib plus cetuximab were fatigue, nausea, diarrhoea, acneiform dermatitis, abdominal pain, arthralgia, decreased appetite, vomiting and rash. The aim of this manuscript is to summarise the scientific review of the application leading to regulatory approval in the EU.
Keywords: BRAF; EMA; V600E; colorectal; encorafenib.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure The authors declare no conflicts of interest.
Figures
References
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Ferlay J., Colombet M., Soerjomataram I. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. - PubMed
-
- Van Cutsem E., Cervantes A., Nordlinger B., Arnold D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii1–iii9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials