Differential modulation of SK channel subtypes by phosphorylation
- PMID: 33422768
- PMCID: PMC8415101
- DOI: 10.1016/j.ceca.2020.102346
Differential modulation of SK channel subtypes by phosphorylation
Abstract
Small-conductance Ca2+-activated K+ (SK) channels are voltage-independent and are activated by Ca2+ binding to the calmodulin constitutively associated with the channels. Both the pore-forming subunits and the associated calmodulin are subject to phosphorylation. Here, we investigated the modulation of different SK channel subtypes by phosphorylation, using the cultured endothelial cells as a tool. We report that casein kinase 2 (CK2) negatively modulates the apparent Ca2+ sensitivity of SK1 and IK channel subtypes by more than 5-fold, whereas the apparent Ca2+ sensitivity of the SK3 and SK2 subtypes is only reduced by ∼2-fold, when heterologously expressed on the plasma membrane of cultured endothelial cells. The SK2 channel subtype exhibits limited cell surface expression in these cells, partly as a result of the phosphorylation of its C-terminus by cyclic AMP-dependent protein kinase (PKA). SK2 channels expressed on the ER and mitochondria membranes may protect against cell death. This work reveals the subtype-specific modulation of the apparent Ca2+ sensitivity and subcellular localization of SK channels by phosphorylation in cultured endothelial cells.
Keywords: Calcium (Ca2+); Calmodulin(CaM); Casein kinase 2 (CK2); Small-conductance Ca2+-activated K+ channels (SK) cyclic AMP-dependent protein kinase (PKA).
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
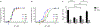
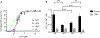
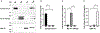
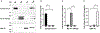


Similar articles
-
Organization and regulation of small conductance Ca2+-activated K+ channel multiprotein complexes.J Neurosci. 2007 Feb 28;27(9):2369-76. doi: 10.1523/JNEUROSCI.3565-06.2007. J Neurosci. 2007. PMID: 17329434 Free PMC article.
-
Protein kinase CK2 is coassembled with small conductance Ca(2+)-activated K+ channels and regulates channel gating.Neuron. 2004 Sep 16;43(6):847-58. doi: 10.1016/j.neuron.2004.08.033. Neuron. 2004. PMID: 15363395
-
PKA phosphorylation underlies functional recruitment of sarcolemmal SK2 channels in ventricular myocytes from hypertrophic hearts.J Physiol. 2020 Jul;598(14):2847-2873. doi: 10.1113/JP277618. Epub 2019 Mar 20. J Physiol. 2020. PMID: 30771223 Free PMC article.
-
Small-conductance calcium-activated potassium channels in the heart: expression, regulation and pathological implications.Philos Trans R Soc Lond B Biol Sci. 2023 Jun 19;378(1879):20220171. doi: 10.1098/rstb.2022.0171. Epub 2023 May 1. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37122223 Free PMC article. Review.
-
Channelopathy of small- and intermediate-conductance Ca2+-activated K+ channels.Acta Pharmacol Sin. 2023 Feb;44(2):259-267. doi: 10.1038/s41401-022-00935-1. Epub 2022 Jun 17. Acta Pharmacol Sin. 2023. PMID: 35715699 Free PMC article. Review.
Cited by
-
Subtype-Selective Positive Modulation of KCa2.3 Channels Increases Cilia Length.ACS Chem Biol. 2022 Aug 19;17(8):2344-2354. doi: 10.1021/acschembio.2c00469. Epub 2022 Aug 10. ACS Chem Biol. 2022. PMID: 35947779 Free PMC article.
-
Loss-of-function KCa2.2 mutations abolish channel activity.Am J Physiol Cell Physiol. 2023 Mar 1;324(3):C658-C664. doi: 10.1152/ajpcell.00584.2022. Epub 2023 Jan 30. Am J Physiol Cell Physiol. 2023. PMID: 36717104 Free PMC article.
-
VTA dopamine neurons are hyperexcitable in 3xTg-AD mice due to casein kinase 2-dependent SK channel dysfunction.Nat Commun. 2024 Nov 8;15(1):9673. doi: 10.1038/s41467-024-53891-1. Nat Commun. 2024. PMID: 39516200 Free PMC article.
-
Casein Kinase 2 Regulates the Intrinsic Activity of L-Type Calcium Currents in Cardiomyocytes.Int J Mol Sci. 2025 Jun 23;26(13):6010. doi: 10.3390/ijms26136010. Int J Mol Sci. 2025. PMID: 40649788 Free PMC article.
-
Asprosin promotes feeding through SK channel-dependent activation of AgRP neurons.Sci Adv. 2023 Feb 22;9(8):eabq6718. doi: 10.1126/sciadv.abq6718. Epub 2023 Feb 22. Sci Adv. 2023. PMID: 36812308 Free PMC article.
References
-
- Adelman JP, Maylie J & Sah P Small-conductance Ca2+-activated K+ channels: Form and function. Annu Rev Physiol 74, 245–269 (2012). - PubMed
-
- Stocker M Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci 5, 758–770 (2004). - PubMed
-
- Kovalevskaya NV et al.Structural analysis of calmodulin binding to ion channels demonstrates the role of its plasticity in regulation. Pflugers Arch (2013). - PubMed
-
- Taylor MS et al.Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res 93, 124–31 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

