Novel cyanothiouracil and cyanothiocytosine derivatives as concentration-dependent selective inhibitors of U87MG glioblastomas: Adenosine receptor binding and potent PDE4 inhibition
- PMID: 33422981
- PMCID: PMC7880896
- DOI: 10.1016/j.ejmech.2020.113125
Novel cyanothiouracil and cyanothiocytosine derivatives as concentration-dependent selective inhibitors of U87MG glioblastomas: Adenosine receptor binding and potent PDE4 inhibition
Abstract
Thiouracil and thiocytosine are important heterocyclic pharmacophores having pharmacological diversity. Antitumor and antiviral activity is commonly associated with thiouracil and thiocytosine derivatives, which are well known fragments for adenosine receptor affinity with many associated pharmacological properties. In this respect, 33 novel compounds have been synthesized in two groups: 24 thiouracil derivatives (4a-x) and 9 thiocytosine derivatives (5a-i). Antitumor activity of all the compounds was determined in the U87 MG glioblastoma cell line. Compound 5e showed an anti-proliferative IC50 of 1.56 μM, which is slightly higher activity than cisplatin (1.67 μM). The 11 most active compounds showed no signficant binding to adenosine A1, A2A or A2B receptors at 1 μM. Brain tumors express high amounts of phosphodiesterases. Compounds were tested for PDE4 inhibition, and 5e and 5f showed the best potency (5e: 3.42 μM; 5f: 0.97 μM). Remakably, those compounds were also the most active against U87MG. However, the compounds lacked a cytotoxic effect on the HEK293 healthy cell line, which encourages further investigation.
Keywords: Adenosine; Antitumor; HEK293; MCF7; PDE; Phosphosdiesterase; Thiocytosine; Thiouracil; U87.
Copyright © 2020 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of competing interest There is not any conflict of interest by the authors.
Figures


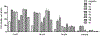


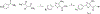

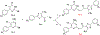
References
-
- Sharma AK, Mahajan MP, Synthesis and [4+2] cycloaddition reactions of 4-(N-allyl-N-aryl)amino-1,3-diaza-1,3-butadienes with vinyl-, isopropenyl- and chloroketenes: Entry to novel pyrimidinone/fused pyrimidinone derivatives., Tetrahedron, 53 (1997) 13841–13854. 10.1016/S0040-4020(97)00873-9. - DOI
-
- Patel A, Pasha Y, Modi A, Synthesis and biological evaluation of 4-aryl substituted −2-(5-carboxylicacid-1, 6-dihydro)-2-thiophenylethylene-6-oxopyrimidine as protein tyrosine phosphatase (PTP-1B) inhibitors, Int. J. Pharmtech. Res, 8 (2015) 136–143. ISSN: 0974–4304.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

