Growth-regulating factor 5 (GRF5)-mediated gene regulatory network promotes leaf growth and expansion in poplar
- PMID: 33423287
- PMCID: PMC8048564
- DOI: 10.1111/nph.17179
Growth-regulating factor 5 (GRF5)-mediated gene regulatory network promotes leaf growth and expansion in poplar
Abstract
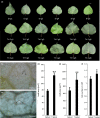
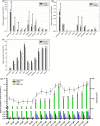
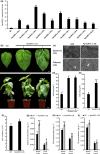
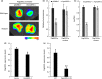
Although polyploid plants have larger leaves than their diploid counterparts, the molecular mechanisms underlying this difference (or trait) remain elusive. Differentially expressed genes (DEGs) between triploid and full-sib diploid poplar trees were identified from two transcriptomic data sets followed by a gene association study among DEGs to identify key leaf growth regulators. Yeast one-hybrid system, electrophoretic mobility shift assay, and dual-luciferase assay were employed to substantiate that PpnGRF5-1 directly regulated PpnCKX1. The interactions between PpnGRF5-1 and growth-regulating factor (GRF)-interacting factors (GIFs) were experimentally validated and a multilayered hierarchical regulatory network (ML-hGRN)-mediated by PpnGRF5-1 was constructed with top-down graphic Gaussian model (GGM) algorithm by combining RNA-sequencing data from its overexpression lines and DAP-sequencing data. PpnGRF5-1 is a negative regulator of PpnCKX1. Overexpression of PpnGRF5-1 in diploid transgenic lines resulted in larger leaves resembling those of triploids, and significantly increased zeatin and isopentenyladenine in the apical buds and third leaves. PpnGRF5-1 also interacted with GIFs to increase its regulatory diversity and capacity. An ML-hGRN-mediated by PpnGRF5-1 was obtained and could largely elucidate larger leaves. PpnGRF5-1 and the ML-hGRN-mediated by PpnGRF5-1 were underlying the leaf growth and development.
Keywords: Populus; cytokinin; gene regulatory network; growth-regulating factor; leaf growth; leaf size; triploid.
© 2021 The Authors. New Phytologist © 2021 New Phytologist Foundation.
Figures




Similar articles
-
Transcriptome comparison of different ploidy reveals the mechanism of photosynthetic efficiency superiority of triploid poplar.Genomics. 2021 Jul;113(4):2211-2220. doi: 10.1016/j.ygeno.2021.05.009. Epub 2021 May 19. Genomics. 2021. PMID: 34022341
-
The interplay of growth-regulating factor 5 and BZR1 in coregulating chlorophyll degradation in poplar.Plant Cell Environ. 2024 Oct;47(10):3766-3779. doi: 10.1111/pce.14958. Epub 2024 May 23. Plant Cell Environ. 2024. PMID: 38783695
-
Ploidy and hybridity effects on leaf size, cell size and related genes expression in triploids, diploids and their parents in Populus.Planta. 2019 Mar;249(3):635-646. doi: 10.1007/s00425-018-3029-0. Epub 2018 Oct 16. Planta. 2019. PMID: 30327883
-
Insights into the Molecular Regulation of Lignin Content in Triploid Poplar Leaves.Int J Mol Sci. 2022 Apr 21;23(9):4603. doi: 10.3390/ijms23094603. Int J Mol Sci. 2022. PMID: 35562994 Free PMC article.
-
MYC2 regulates stomatal density and water use efficiency via targeting EPF2/EPFL4/EPFL9 in poplar.New Phytol. 2024 Mar;241(6):2506-2522. doi: 10.1111/nph.19531. Epub 2024 Jan 23. New Phytol. 2024. PMID: 38258389
Cited by
-
Functional Mechanisms and the Application of Developmental Regulators for Improving Genetic Transformation in Plants.Plants (Basel). 2024 Oct 10;13(20):2841. doi: 10.3390/plants13202841. Plants (Basel). 2024. PMID: 39458788 Free PMC article. Review.
-
Characterization and genetic differences analysis in adventitious roots development of 38 Populus germplasm resources.Plant Mol Biol. 2024 Feb 5;114(1):9. doi: 10.1007/s11103-024-01418-z. Plant Mol Biol. 2024. PMID: 38315324
-
Transcriptomic Complexity of Culm Growth and Development in Different Types of Moso Bamboo.Int J Mol Sci. 2023 Apr 18;24(8):7425. doi: 10.3390/ijms24087425. Int J Mol Sci. 2023. PMID: 37108588 Free PMC article. Review.
-
Breeding polyploid Populus: progress and perspective.For Res (Fayettev). 2022 Mar 31;2:4. doi: 10.48130/FR-2022-0004. eCollection 2022. For Res (Fayettev). 2022. PMID: 39525419 Free PMC article. Review.
-
Identification of gene family members and a key structural variation reveal important roles of OVATE genes in regulating tea (Camellia sinensis) leaf development.Front Plant Sci. 2022 Sep 23;13:1008408. doi: 10.3389/fpls.2022.1008408. eCollection 2022. Front Plant Sci. 2022. PMID: 36212328 Free PMC article.
References
-
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological) 57: 289–300.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

