Coronavirus disease 2019 pandemic and allogeneic hematopoietic stem cell transplantation: a single center reappraisal
- PMID: 33423867
- PMCID: PMC7732233
- DOI: 10.1016/j.jcyt.2020.12.001
Coronavirus disease 2019 pandemic and allogeneic hematopoietic stem cell transplantation: a single center reappraisal
Abstract
Background: The coronavirus disease 2019 (COVID-19) pandemic has deeply modified the complex logistical process underlying allogeneic hematopoietic stem cell transplant practices.
Aim: In light of these changes, the authors compared data relative to allogeneic transplants carried out from 2018 at their center before (n = 167) and during the pandemic (n = 45).
Methods: The authors examined patient characteristics, donor and graft types, cell doses and main transplant outcomes. Moreover, the authors evaluated the rise of costs attributable to additional COVID-19-related procedures as well as the risk of adverse events these procedures conveyed to grafts or recipients.
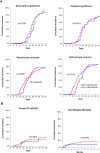
Results: Overall, the number of transplants did not decrease during the pandemic, whereas patients at high relapse risk were prioritized. Transplants were mainly from matched unrelated donors, with a significant decrease in haploidentical related donors. Moreover, the use of bone marrow as a graft for haploidentical transplant was almost abandoned. Cryopreservation was introduced for all related and unrelated apheresis products, with a median storage time of 20 days. Notably, transplant outcomes (engraftment, acute graft-versus-host disease and non-relapse mortality) with cryopreserved products were comparable to those with fresh products.
Conclusions: Considering that the emergency situation may persist for months, cryopreserving allogeneic grafts can offer a lifesaving opportunity for patients whose allogeneic transplant cannot be postponed until after the end of the COVID-19 pandemic.
Keywords: COVID-19 pandemic; allogeneic hematopoietic stem cell transplantation; costs; cryopreservation.
Copyright © 2020 International Society for Cell & Gene Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have no commercial, proprietary or financial interest in the products or companies described in this article.
Figures
References
-
- http://www.trapianti.salute.gov.it/imgs/C_17_cntAvvisi_227_0_file.pdf (last accessed on November 16, 2020).
-
- http://www.trapianti.salute.gov.it/imgs/C_17_cntAvvisi_225_0_file.pdf (last accessed on November 16, 2020).
-
- http://www.trapianti.salute.gov.it/imgs/C_17_cntAvvisi_236_0_file.pdf (last accessed on November 16, 2020).
-
- Ljungman P, Mikulska M, de la Camara R, Basak GW, Chabannon C, Corbacioglu S. Correction: The challenge of COVID-19 and hematopoietic cell transplantation: EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020;8:1. - PMC - PubMed
-
- Algwaiz G, Aljurf M, Koh M, Horowitz MM, Ljungman P, Weisdorf D. Real-World Issues and Potential Solutions in Hematopoietic Cell Transplantation during the COVID-19 Pandemic: Perspectives from the Worldwide Network for Blood and Marrow Transplantation and Center for International Blood and Marrow Transplant Research Health Services and International Studies Committee. Biol Blood Marrow Transplant. 2020;26(12):2181–2189. doi: 10.1016/j.bbmt.2020.07.021. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

