A robust unsupervised machine-learning method to quantify the morphological heterogeneity of cells and nuclei
- PMID: 33424024
- PMCID: PMC8167883
- DOI: 10.1038/s41596-020-00432-x
A robust unsupervised machine-learning method to quantify the morphological heterogeneity of cells and nuclei
Abstract
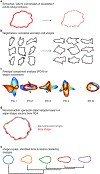
Cell morphology encodes essential information on many underlying biological processes. It is commonly used by clinicians and researchers in the study, diagnosis, prognosis, and treatment of human diseases. Quantification of cell morphology has seen tremendous advances in recent years. However, effectively defining morphological shapes and evaluating the extent of morphological heterogeneity within cell populations remain challenging. Here we present a protocol and software for the analysis of cell and nuclear morphology from fluorescence or bright-field images using the VAMPIRE algorithm ( https://github.com/kukionfr/VAMPIRE_open ). This algorithm enables the profiling and classification of cells into shape modes based on equidistant points along cell and nuclear contours. Examining the distributions of cell morphologies across automatically identified shape modes provides an effective visualization scheme that relates cell shapes to cellular subtypes based on endogenous and exogenous cellular conditions. In addition, these shape mode distributions offer a direct and quantitative way to measure the extent of morphological heterogeneity within cell populations. This protocol is highly automated and fast, with the ability to quantify the morphologies from 2D projections of cells seeded both on 2D substrates or embedded within 3D microenvironments, such as hydrogels and tissues. The complete analysis pipeline can be completed within 60 minutes for a dataset of ~20,000 cells/2,400 images.
Figures




References
-
- Bakal C, Aach J, Church G & Perrimon N Quantitative morphological signatures define local signaling networks regulating cell morphology. Science 316, 1753–1756 (2007). - PubMed
Publication types
MeSH terms
Grants and funding
- U54 CA210173/CA/NCI NIH HHS/United States
- R01CA174388/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- P30 AG021334/AG/NIA NIH HHS/United States
- U01 AG060903/AG/NIA NIH HHS/United States
- U01AG060903/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
LinkOut - more resources
Full Text Sources
Other Literature Sources

