Marine biomimetics: bromotyrosines loaded chitinous skeleton as source of antibacterial agents
- PMID: 33424135
- PMCID: PMC7776313
- DOI: 10.1007/s00339-020-04167-0
Marine biomimetics: bromotyrosines loaded chitinous skeleton as source of antibacterial agents
Abstract
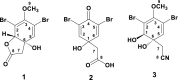
The marine sponges of the order Verongiida (Demospongiae: Porifera) have survived on our planet for more than 500 million years due to the presence of a unique strategy of chemical protection by biosynthesis of more than 300 derivatives of biologically active bromotyrosines as secondary metabolites. These compounds are synthesized within spherulocytes, highly specialized cells located within chitinous skeletal fibers of these sponges from where they can be extruded in the sea water and form protective space against pathogenic viruses, bacteria and other predators. This chitin is an example of unique biomaterial as source of substances with antibiotic properties. Traditionally, the attention of researchers was exclusively drawn to lipophilic bromotyrosines, the extraction methods of which were based on the use of organic solvents only. Alternatively, we have used in this work a biomimetic water-based approach, because in natural conditions, sponges actively extrude bromotyrosines that are miscible with the watery environment. This allowed us to isolate 3,5-dibromoquinolacetic acid from an aqueous extract of the dried demosponge Aplysina aerophoba and compare its antimicrobial activity with the same compound obtained by the chemical synthesis. Both synthetic and natural compounds have shown antimicrobial properties against clinical strains of Staphylococcus aureus, Enterococcus faecalis and Propionibacterium acnes.
Supplementary information: The online version contains supplementary material available at 10.1007/s00339-020-04167-0.
Keywords: Antibacterial; Biomimetics; Bromotyrosines; Chitin; Secondary metabolites; Spherulocytes; Sponges.
© Springer-Verlag GmbH Germany, part of Springer Nature 2021.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures



Similar articles
-
On the Mechanical Properties of Microfibre-Based 3D Chitinous Scaffolds from Selected Verongiida Sponges.Mar Drugs. 2023 Aug 24;21(9):463. doi: 10.3390/md21090463. Mar Drugs. 2023. PMID: 37755076 Free PMC article.
-
The Loss of Structural Integrity of 3D Chitin Scaffolds from Aplysina aerophoba Marine Demosponge after Treatment with LiOH.Mar Drugs. 2023 May 30;21(6):334. doi: 10.3390/md21060334. Mar Drugs. 2023. PMID: 37367659 Free PMC article.
-
Express Method for Isolation of Ready-to-Use 3D Chitin Scaffolds from Aplysina archeri (Aplysineidae: Verongiida) Demosponge.Mar Drugs. 2019 Feb 22;17(2):131. doi: 10.3390/md17020131. Mar Drugs. 2019. PMID: 30813373 Free PMC article.
-
Bacteria From Marine Sponges: A Source of New Drugs.Curr Drug Metab. 2017;18(1):11-15. doi: 10.2174/1389200217666161013090610. Curr Drug Metab. 2017. PMID: 27739371 Review.
-
Sponge derived bromotyrosines: structural diversity through natural combinatorial chemistry.Nat Prod Commun. 2015 Jan;10(1):219-31. Nat Prod Commun. 2015. PMID: 25920247 Review.
Cited by
-
Biomaterials from the sea: Future building blocks for biomedical applications.Bioact Mater. 2021 Apr 29;6(12):4255-4285. doi: 10.1016/j.bioactmat.2021.04.028. eCollection 2021 Dec. Bioact Mater. 2021. PMID: 33997505 Free PMC article. Review.
-
The Anti-Viral Applications of Marine Resources for COVID-19 Treatment: An Overview.Mar Drugs. 2021 Jul 23;19(8):409. doi: 10.3390/md19080409. Mar Drugs. 2021. PMID: 34436248 Free PMC article. Review.
-
On the Mechanical Properties of Microfibre-Based 3D Chitinous Scaffolds from Selected Verongiida Sponges.Mar Drugs. 2023 Aug 24;21(9):463. doi: 10.3390/md21090463. Mar Drugs. 2023. PMID: 37755076 Free PMC article.
-
Calcite Nanotuned Chitinous Skeletons of Giant Ianthella basta Marine Demosponge.Int J Mol Sci. 2021 Nov 22;22(22):12588. doi: 10.3390/ijms222212588. Int J Mol Sci. 2021. PMID: 34830470 Free PMC article.
-
The Loss of Structural Integrity of 3D Chitin Scaffolds from Aplysina aerophoba Marine Demosponge after Treatment with LiOH.Mar Drugs. 2023 May 30;21(6):334. doi: 10.3390/md21060334. Mar Drugs. 2023. PMID: 37367659 Free PMC article.
References
-
- Bergquist PR, de Cook SC. Syst Porifera. Berlin: Springer; 2002. p. 1081.
-
- Ehrlich H, Simon P, Carrillo-Cabrera W, Bazhenov VV, Botting JP, Ilan M, Ereskovsky AV, Muricy G, Worch H, Mensch A, Born R, Springer A, Kummer K, Vyalikh DV, Molodtsov SL, Kurek D, Kammer M, Paasch S, Brunner E. Chem. Mater. 2010;22:1462. doi: 10.1021/cm9026607. - DOI
-
- Ehrlich H, Steck E, Ilan M, Maldonado M, Muricy G, Bavestrello G, Kljajic Z, Carballo JL, Schiaparelli S, Ereskovsky A, Schupp P, Born R, Worch H, Bazhenov VV, Kurek D, Varlamov V, Vyalikh D, Kummer K, Sivkov VV, Molodtsov SL, Meissner H, Richter G, Hunoldt S, Kammer M, Paasch S, Krasokhin V, Patzke G, Brunner E, Richter W. Int. J. Biol. Macromol. 2010;47:141. doi: 10.1016/j.ijbiomac.2010.05.009. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
