Roles of oxidative stress, apoptosis, and inflammation in metal-induced dysfunction of beta pancreatic cells isolated from CD1 mice
- PMID: 33424352
- PMCID: PMC7785459
- DOI: 10.1016/j.sjbs.2020.10.056
Roles of oxidative stress, apoptosis, and inflammation in metal-induced dysfunction of beta pancreatic cells isolated from CD1 mice
Abstract
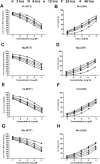
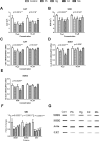
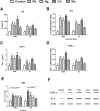
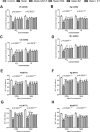
The diabetogenic effects of metals including lead (Pb), mercury (Hg), cadmium (Cd), and molybdenum (Mo) have been reported with poorly identified underlying mechanisms. The current study assessed the effect of metals on the roles of oxidative stress, apoptosis, and inflammation in beta pancreatic cells isolated from CD-1 mice, via different biochemical assays. Data showed that the tested metals were cytotoxic to the isolated cells with impaired glucose stimulated insulin secretion (GSIS). This was associated with increased reactive oxygen species (ROS) production, lipid peroxidation, antioxidant enzymes activities, active proapoptotic caspase-3 (cas-3), inflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) levels in the intoxicated cells. Furthermore, antioxidant-reduced glutathione (GSH-R), cas-3 inhibitor z-VAD-FMK, IL-6 inhibitor bazedoxifene (BZ), and TNF-α inhibitor etanercept (ET) were found to significantly decrease metal-induced cytotoxicity with improved GSIS in metals' intoxicated cells. In conclusion, oxidative stress, apoptosis, and inflammation can play roles in metals-induced diabetogenic effect.
Keywords: Cadmium; Inflammation; Lead; Mercury; Molybdenum; Oxidative stress; Pancreatic beta cells.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Assessment of antipsychotic-induced cytotoxic effects on isolated CD1 mouse pancreatic beta cells.Int J Health Sci (Qassim). 2023 Jul-Aug;17(4):11-21. Int J Health Sci (Qassim). 2023. PMID: 37416840 Free PMC article.
-
Effects of environmental metals on mitochondrial bioenergetics of the CD-1 mice pancreatic beta-cells.Toxicol In Vitro. 2021 Feb;70:105015. doi: 10.1016/j.tiv.2020.105015. Epub 2020 Oct 8. Toxicol In Vitro. 2021. PMID: 33038468
-
z-VAD-fmk augmentation of TNF alpha-stimulated neutrophil apoptosis is compound specific and does not involve the generation of reactive oxygen species.Blood. 2005 Apr 1;105(7):2970-2. doi: 10.1182/blood-2004-07-2870. Epub 2004 Nov 30. Blood. 2005. PMID: 15572588
-
Heavy-metal-induced reactive oxygen species: phytotoxicity and physicochemical changes in plants.Rev Environ Contam Toxicol. 2014;232:1-44. doi: 10.1007/978-3-319-06746-9_1. Rev Environ Contam Toxicol. 2014. PMID: 24984833 Review.
-
Mitochondrial Oxidative Stress Is the General Reason for Apoptosis Induced by Different-Valence Heavy Metals in Cells and Mitochondria.Int J Mol Sci. 2023 Sep 22;24(19):14459. doi: 10.3390/ijms241914459. Int J Mol Sci. 2023. PMID: 37833908 Free PMC article. Review.
Cited by
-
Trace elements in pancreatic cancer.Cancer Med. 2024 Jul;13(14):e7454. doi: 10.1002/cam4.7454. Cancer Med. 2024. PMID: 39015024 Free PMC article. Review.
-
Potential of oligonucleotide- and protein/peptide-based therapeutics in the management of toxicant/stressor-induced diseases.Naunyn Schmiedebergs Arch Pharmacol. 2024 Mar;397(3):1275-1310. doi: 10.1007/s00210-023-02683-3. Epub 2023 Sep 9. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37688622 Review.
-
Persistence of improved glucose homeostasis in Gclm null mice with age and cadmium treatment.Redox Biol. 2022 Feb;49:102213. doi: 10.1016/j.redox.2021.102213. Epub 2021 Dec 20. Redox Biol. 2022. PMID: 34953454 Free PMC article.
-
Genetic and epigenetic modulations in toxicity: The two-sided roles of heavy metals and polycyclic aromatic hydrocarbons from the environment.Toxicol Rep. 2024 May 4;12:502-519. doi: 10.1016/j.toxrep.2024.04.010. eCollection 2024 Jun. Toxicol Rep. 2024. PMID: 38774476 Free PMC article. Review.
-
Association between Heavy metals and triglyceride-glucose-related index: a mediation analysis of inflammation indicators.Lipids Health Dis. 2025 Feb 13;24(1):46. doi: 10.1186/s12944-025-02441-9. Lipids Health Dis. 2025. PMID: 39948676 Free PMC article.
References
-
- Ajibola R.S., Ogundahunsi O.A., Soyinka O.O., Ogunyemi E.O., Odewabi A.O. Serum chromium, molybdenum, zinc and magnesium levels in diabetes mellitus patients in Sagamu, South West Nigeria. Asian J. Med. Sci. 2014;6(2):15–19. doi: 10.19026/ajms.6.5350. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

