Target Molecules of STIM Proteins in the Central Nervous System
- PMID: 33424550
- PMCID: PMC7786003
- DOI: 10.3389/fnmol.2020.617422
Target Molecules of STIM Proteins in the Central Nervous System
Abstract
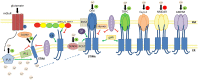
Stromal interaction molecules (STIMs), including STIM1 and STIM2, are single-pass transmembrane proteins that are located predominantly in the endoplasmic reticulum (ER). They serve as calcium ion (Ca2+) sensors within the ER. In the central nervous system (CNS), they are involved mainly in Orai-mediated store-operated Ca2+ entry (SOCE). The key molecular components of the SOCE pathway are well-characterized, but the molecular mechanisms that underlie the regulation of this pathway need further investigation. Numerous intracellular target proteins that are located in the plasma membrane, ER, cytoskeleton, and cytoplasm have been reported to play essential roles in concert with STIMs, such as conformational changes in STIMs, their translocation, the stabilization of their interactions with Orai, and the activation of other channels. The present review focuses on numerous regulators, such as Homer, SOCE-associated regulatory factor (SARAF), septin, synaptopodin, golli proteins, partner of STIM1 (POST), and transcription factors and proteasome inhibitors that regulate STIM-Orai interactions in the CNS. Further we describe novel roles of STIMs in mediating Ca2+ influx via other than Orai pathways, including TRPC channels, VGCCs, AMPA and NMDA receptors, and group I metabotropic glutamate receptors. This review also summarizes recent findings on additional molecular targets of STIM proteins including SERCA, IP3Rs, end-binding proteins (EB), presenilin, and CaMKII. Dysregulation of the SOCE-associated toolkit, including STIMs, contributes to the development of neurodegenerative disorders (e.g., Alzheimer's disease, Parkinson's disease, and Huntington's disease), traumatic brain injury, epilepsy, and stroke. Emerging evidence points to the role of STIM proteins and several of their molecular effectors and regulators in neuronal and glial physiology and pathology, suggesting their potential application for future therapeutic strategies.
Keywords: Ca2+ channels; SOCE components; STIM; STIM regulators and effectors; calcium signaling; central nervous system; glutamate receptors; store-operated calcium entry (SOCE).
Copyright © 2020 Serwach and Gruszczynska-Biegala.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Agrawal N., Venkiteswaran G., Sadaf S., Padmanabhan N., Banerjee S., Hasan G. (2010). Inositol 1,4,5-trisphosphate receptor and dSTIM function in Drosophila insulin-producing neurons regulates systemic intracellular calcium homeostasis and flight. J. Neurosci. 30, 1301–1313. 10.1523/JNEUROSCI.3668-09.2010 - DOI - PMC - PubMed
-
- Albarran L., Lopez J. J., Woodard G. E., Salido G. M., Rosado J. A. (2016). Store-operated Ca2+ entry-associated regulatory factor (SARAF) plays an important role in the regulation of arachidonate-regulated Ca2+ (ARC) channels. J Biol Chem. 291, 6982–6988. 10.1074/jbc.M115.704940 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

