AAV-Mediated GALC Gene Therapy Rescues Alpha-Synucleinopathy in the Spinal Cord of a Leukodystrophic Lysosomal Storage Disease Mouse Model
- PMID: 33424556
- PMCID: PMC7785790
- DOI: 10.3389/fncel.2020.619712
AAV-Mediated GALC Gene Therapy Rescues Alpha-Synucleinopathy in the Spinal Cord of a Leukodystrophic Lysosomal Storage Disease Mouse Model
Abstract
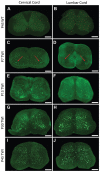
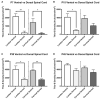
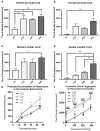
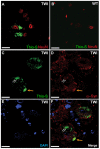
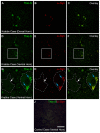
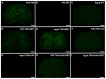
Krabbe's disease (KD) is primarily a demyelinating disorder, but recent studies have identified the presence of neuronal protein aggregates in the brain, at least partially composed by alpha-synuclein (α-syn). The role of this protein aggregation in the pathogenesis of KD is largely unknown, but it has added KD to a growing list of lysosomal storage diseases that can be also be considered as proteinopathies. While the presence of these protein aggregates within the KD brain is now appreciated, the remainder of the central nervous system (CNS) remains uncharacterized. This study is the first to report the presence of thioflavin-S reactive inclusions throughout the spinal cord of both murine and human spinal tissue. Stereological analysis revealed the temporal and spatial accumulation of these inclusions within the neurons of the ventral spinal cord vs. those located in the dorsal cord. This study also confirmed that these thio-S positive accumulations are present within neuronal populations and are made up at least in part by α-syn in both the twitcher mouse and cord autopsied material from affected human patients. Significantly, neonatal gene therapy for galactosylceramidase, a treatment that strongly improves the survival and health of KD mice, but not bone marrow transplantation prevents the formation of these inclusions in spinal neurons. These results expand the understanding of α-syn protein aggregation within the CNS of individuals afflicted with KD and underlines the tractability of this problem via early gene therapy, with potential impact to other synucleinopathies such as PD.
Keywords: Parkinson's disease; alpha-synuclein; globoid cell leukodystrophy; proteinopathies; spinal cord.
Copyright © 2020 Marshall, Issa, Heller, Nguyen and Bongarzone.
Conflict of interest statement
EB is a consultant for Lysosomal Therapeutics Inc., E-Scape Bio, Gain Therapeutics, Affinia, and Neurogene. Neither entity provided support in the form of salaries for any listed author nor played additional roles in the study design, data collection, analysis, decision to publish, or preparation of the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

