Endocannabinoid-Like Lipid Neuromodulators in the Regulation of Dopamine Signaling: Relevance for Drug Addiction
- PMID: 33424577
- PMCID: PMC7786397
- DOI: 10.3389/fnsyn.2020.588660
Endocannabinoid-Like Lipid Neuromodulators in the Regulation of Dopamine Signaling: Relevance for Drug Addiction
Abstract
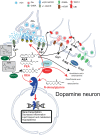
The family of lipid neuromodulators has been rapidly growing, as the use of different -omics techniques led to the discovery of a large number of naturally occurring N-acylethanolamines (NAEs) and N-acyl amino acids belonging to the complex lipid signaling system termed endocannabinoidome. These molecules exert a variety of biological activities in the central nervous system, as they modulate physiological processes in neurons and glial cells and are involved in the pathophysiology of neurological and psychiatric disorders. Their effects on dopamine cells have attracted attention, as dysfunctions of dopamine systems characterize a range of psychiatric disorders, i.e., schizophrenia and substance use disorders (SUD). While canonical endocannabinoids are known to regulate excitatory and inhibitory synaptic inputs impinging on dopamine cells and modulate several dopamine-mediated behaviors, such as reward and addiction, the effects of other lipid neuromodulators are far less clear. Here, we review the emerging role of endocannabinoid-like neuromodulators in dopamine signaling, with a focus on non-cannabinoid N-acylethanolamines and their receptors. Mounting evidence suggests that these neuromodulators contribute to modulate synaptic transmission in dopamine regions and might represent a target for novel medications in alcohol and nicotine use disorder.
Keywords: N-acylethanolamines; alcohol; dopamine neurons; endocannabinoids; nicotine; peroxisome proliferator-activated receptors-α.
Copyright © 2020 Sagheddu, Torres, Marcourakis and Pistis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Alen F., Decara J., Brunori G., You Z.-B., Bühler K.-M., López-Moreno J. A., et al. . (2018). PPARα/CB1 receptor dual ligands as a novel therapy for alcohol use disorder: evaluation of a novel oleic acid conjugate in preclinical rat models. Biochem. Pharmacol. 157, 235–243. 10.1016/j.bcp.2018.09.008 - DOI - PubMed
-
- Auboeuf D., Rieusset J., Fajas L., Vallier P., Frering V., Riou J. P., et al. . (1997). Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-α in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes 46, 1319–1327. 10.2337/diab.46.8.1319 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

