Structural and Functional Plasticity in the Regenerating Olfactory System of the Migratory Locust
- PMID: 33424632
- PMCID: PMC7793960
- DOI: 10.3389/fphys.2020.608661
Structural and Functional Plasticity in the Regenerating Olfactory System of the Migratory Locust
Abstract
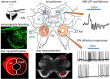
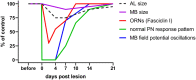
Regeneration after injury is accompanied by transient and lasting changes in the neuroarchitecture of the nervous system and, thus, a form of structural plasticity. In this review, we introduce the olfactory pathway of a particular insect as a convenient model to visualize neural regeneration at an anatomical level and study functional recovery at an electrophysiological level. The olfactory pathway of the locust (Locusta migratoria) is characterized by a multiglomerular innervation of the antennal lobe by olfactory receptor neurons. These olfactory afferents were axotomized by crushing the base of the antenna. The resulting degeneration and regeneration in the antennal lobe could be quantified by size measurements, dye labeling, and immunofluorescence staining of cell surface proteins implicated in axonal guidance during development. Within 3 days post lesion, the antennal lobe volume was reduced by 30% and from then onward regained size back to normal by 2 weeks post injury. The majority of regenerating olfactory receptor axons reinnervated the glomeruli of the antennal lobe. A few regenerating axons project erroneously into the mushroom body on a pathway that is normally chosen by second-order projection neurons. Based on intracellular responses of antennal lobe output neurons to odor stimulation, regenerated fibers establish functional synapses again. Following complete absence after nerve crush, responses to odor stimuli return to control level within 10-14 days. On average, regeneration of afferents, and re-established synaptic connections appear faster in younger fifth instar nymphs than in adults. The initial degeneration of olfactory receptor axons has a trans-synaptic effect on a second order brain center, leading to a transient size reduction of the mushroom body calyx. Odor-evoked oscillating field potentials, absent after nerve crush, were restored in the calyx, indicative of regenerative processes in the network architecture. We conclude that axonal regeneration in the locust olfactory system appears to be possible, precise, and fast, opening an avenue for future mechanistic studies. As a perspective of biomedical importance, the current evidence for nitric oxide/cGMP signaling as positive regulator of axon regeneration in connectives of the ventral nerve cord is considered in light of particular regeneration studies in vertebrate central nervous systems.
Keywords: antennal lobe; fasciclin I; field potential oscillations; mushroom body; semaphorin 1a.
Copyright © 2020 Bicker and Stern.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Anton S., Hansson B. S. (1996). Antennal lobe interneurons in the desert locust Schistocerca gregaria (Forskal): processing of aggregation pheromones in adult males and females. J. Comp. Neurol. 370 85–96. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

