The Body Weight Alteration and Incidence of Neoplasm in Patients With Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials
- PMID: 33424764
- PMCID: PMC7793753
- DOI: 10.3389/fendo.2020.541699
The Body Weight Alteration and Incidence of Neoplasm in Patients With Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials
Abstract
Objective: Whether hypoglycemic treatments with weight-alternating effects influence the incidence of neoplasm in type 2 diasbetes (T2D) remains uncertain. Therefore, we performed a meta-analysis to assess the association between the weight alteration and incidence of neoplasm in patients with T2D.
Research design and methods: Systematic searches were conducted for studies published between the inception of 1950s and September 2019. Randomized controlled trials conducted in T2D patients with at least 48-week follow-up, significant weight change difference between treatment arms and reports of neoplasm events were included. Fixed-effects model and meta-regression analysis were accordingly used.
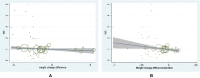
Results: In all, 46 studies were included. Analysis indicated weight reduction was not associated with a decreased incidence of neoplasm (OR = 1.01, 95% CI, 0.96 to 1.07, I2 = 17%) and weight elevation was not associated with an increased incidence of neoplasm (OR = 0.91, 95% CI, 0.76 to 1.09, I2 = 0%). Meta-regression analysis showed a slower weight reduction rate (β = -5.983, 95% CI, -11.412 to 0.553, P = 0.03) instead of weight change difference (β = -0.030, 95% CI, -0.068 to 0.007, P = 0.115) was significantly associated with reduced risk of neoplasm in patients with T2D. Moreover, a decreased incidence of prostate, bladder, and uterine neoplasm was observed in T2D patients with weight reduction difference while an increased incidence of thyroid neoplasm was found in glucagon-like peptide-1 receptor analog (GLP-1RA) users with weight reduction difference.
Conclusions: Additional weight change achieved by current hypoglycemic agents or strategies in short and medium periods was not associated with incidence of most neoplasm in patients with T2D. However, a decreased incidence of prostate, bladder, and uterine neoplasm was shown in T2D patients with weight reduction difference while an increased risk of thyroid neoplasm was observed in T2D patients on GLP-1RA treatments with weight reduction difference. A more sustained and persistent weight reduction process may confer reduced risk of neoplasm in patients with T2D.
Keywords: body-weight trajectory; cancer; hypoglycemic agents; neoplasms; type 2 diabetes.
Copyright © 2020 Lin, Cai, Yang, Lv, Nie and Ji.
Conflict of interest statement
LJ has received fees for lecture presentations and for consulting from AstraZeneca, Merck, Metabasis, MSD, Novartis, Eli Lilly, Roche, Sanofi-Aventis, and Takeda. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Glycemic control and the incidence of neoplasm in patients with type 2 diabetes: a meta-analysis of randomized controlled trials.Endocrine. 2020 Nov;70(2):232-242. doi: 10.1007/s12020-020-02376-4. Epub 2020 Jun 12. Endocrine. 2020. PMID: 32533507
-
GLP-1 receptor agonist added to insulin versus basal-plus or basal-bolus insulin therapy in type 2 diabetes: A systematic review and meta-analysis.Diabetes Metab Res Rev. 2019 Jan;35(1):e3082. doi: 10.1002/dmrr.3082. Epub 2018 Oct 18. Diabetes Metab Res Rev. 2019. PMID: 30270567
-
Effects of glucagon-like peptide-1 receptor agonists on major cardiovascular events in patients with Type 2 diabetes mellitus with or without established cardiovascular disease: a meta-analysis of randomized controlled trials.Eur Heart J. 2020 Sep 14;41(35):3346-3358. doi: 10.1093/eurheartj/ehaa082. Eur Heart J. 2020. PMID: 32077924
-
Association of glucagon-like peptide-1 receptor agonist use and rates of acute myocardial infarction, stroke and overall mortality in patients with type 2 diabetes mellitus in a large integrated health system.Diabetes Obes Metab. 2017 Nov;19(11):1555-1561. doi: 10.1111/dom.12969. Epub 2017 Jul 5. Diabetes Obes Metab. 2017. PMID: 28407414
-
Glycemic efficacy and safety of glucagon-like peptide-1 receptor agonist on top of sodium-glucose co-transporter-2 inhibitor treatment compared to sodium-glucose co-transporter-2 inhibitor alone: A systematic review and meta-analysis of randomized controlled trials.Diabetes Res Clin Pract. 2019 Dec;158:107927. doi: 10.1016/j.diabres.2019.107927. Epub 2019 Nov 13. Diabetes Res Clin Pract. 2019. PMID: 31733280
Cited by
-
Relationship between the Plasma Proteome and Changes in Inflammatory Markers after Bariatric Surgery.Cells. 2021 Oct 19;10(10):2798. doi: 10.3390/cells10102798. Cells. 2021. PMID: 34685777 Free PMC article.
-
Effect of glucagon-like peptide-1 receptor agonists on prostate cancer: A review.Medicine (Baltimore). 2024 Oct 11;103(41):e39956. doi: 10.1097/MD.0000000000039956. Medicine (Baltimore). 2024. PMID: 39465848 Free PMC article. Review.
-
Glycosylated haemoglobin and prognosis in 10,536 people with cancer and pre-existing diabetes: a meta-analysis with dose-response analysis.BMC Cancer. 2022 Oct 6;22(1):1048. doi: 10.1186/s12885-022-10144-y. BMC Cancer. 2022. PMID: 36203139 Free PMC article.
-
Association of serum fetuin-B with insulin resistance and pre-diabetes in young Chinese women: evidence from a cross-sectional study and effect of liraglutide.PeerJ. 2021 Aug 20;9:e11869. doi: 10.7717/peerj.11869. eCollection 2021. PeerJ. 2021. PMID: 34484983 Free PMC article. Clinical Trial.
-
Changes in the Proteome Profile of People Achieving Remission of Type 2 Diabetes after Bariatric Surgery.J Clin Med. 2021 Aug 18;10(16):3659. doi: 10.3390/jcm10163659. J Clin Med. 2021. PMID: 34441954 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

