Risk Factors for Adverse Neurodevelopment in Transient or Persistent Congenital Hyperinsulinism
- PMID: 33424766
- PMCID: PMC7793856
- DOI: 10.3389/fendo.2020.580642
Risk Factors for Adverse Neurodevelopment in Transient or Persistent Congenital Hyperinsulinism
Abstract
Objective: Aim was to identify hypotheses why adverse neurodevelopment still occurs in children with transient or persistent hyperinsulinism despite improvements in long-term treatment options during the last decades.
Material and methods: A retrospective review of 87 children with transient (n=37) or persistent congenital hyperinsulinism (CHI) (n=50) was conducted at the University Children's Hospital Duesseldorf, Germany. Possible risk factors for neurodevelopmental sequelae due to hypoglycemia were analyzed with a focus on the first days after onset of disease.
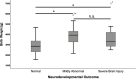
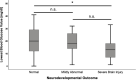
Results: Median age at follow-up was 7 years (IQR 8). Adverse neurodevelopmental outcome was seen in 34.5% (n=30) of all CHI patients. Fifteen had mildly abnormal neurodevelopment and 15 had a severe hypoglycemic brain injury. In univariate analysis, mildly abnormal neurodevelopment was associated with the diagnosis of persistent CHI (odds ratio (OR) 8.3; p=0.004) and higher birth weight (mean difference 1049 g; p<0.001). Severe hypoglycemic brain injury was associated with the diagnosis of persistent CHI (OR 5.1; p=0.013), being born abroad (OR 18.3; p<0.001) or in a lower-level maternity hospital (OR 4.8; p=0.039), and of note history of hypoglycemic seizures (OR 13.0; p=<0.001), and a delay between first symptoms of hypoglycemia and first blood glucose measurement/initiation of treatment (OR 10.7; p<0.001). Children with severe hypoglycemic brain injury had lower recorded blood glucose (mean difference -8.34 mg/dl; p=0.022) and higher birth weight than children with normal development (mean difference 829 g; p=0.012). In multivariate binary logistic regression models, lowest blood glucose <20 mg/dl (OR 134.3; p=0.004), a delay between initial symptoms and first blood glucose measurement/initiation of treatment (OR 71.7; p=0.017) and hypoglycemic seizures (OR 12.9; p=0.008) were positively correlated with severe brain injury. Analysis showed that the odds for brain injury decreased by 15% (OR 0.85; p=0.035) if the blood glucose increased by one unit.
Conclusion: While some risk factors for adverse outcome in CHI are not influenceable, others like lowest recorded blood glucose values <20 mg/dl, hypoglycemic seizures, and insufficiently-or even untreated hypoglycemia can be avoided. Future guidelines for management of neonatal hypoglycemia should address this by ensuring early identification and immediate treatment with appropriate escalation steps.
Keywords: brain injury; hyperinsulinism; hypoglycemia; neurodevelopment; outcome; risk factors.
Copyright © 2020 Roeper, Salimi Dafsari, Hoermann, Mayatepek, Kummer and Meissner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

