Glucocorticoid-Induced Leucine Zipper: A Promising Marker for Monitoring and Treating Sepsis
- PMID: 33424852
- PMCID: PMC7793647
- DOI: 10.3389/fimmu.2020.606649
Glucocorticoid-Induced Leucine Zipper: A Promising Marker for Monitoring and Treating Sepsis
Abstract
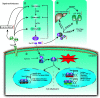
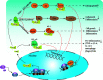
Sepsis is a clinical syndrome that resulting from a dysregulated inflammatory response to infection that leads to organ dysfunction. The dysregulated inflammatory response transitions from a hyper-inflammatory phase to a hypo-inflammatory or immunosuppressive phase. Currently, no phase-specific molecular-based therapies are available for monitoring the complex immune response and treating sepsis due to individual variations in the timing and overlap of the dysregulated immune response in most patients. Glucocorticoid-induced leucine zipper (GILZ), is broadly present in multiple tissues and circumvent glucocorticoid resistance (GCR) or unwanted side effects. Recently, the characteristics of GILZ downregulation during acute hyperinflammation and GILZ upregulation during the immunosuppressive phase in various inflammatory diseases have been well documented, and the protective effects of GILZ have gained attention in the field of sepsis. However, whether GILZ could be a promising candidate biomarker for monitoring and treating septic patients remains unknown. Here, we discuss the effect of GILZ in sepsis and sepsis-induced immunosuppression.
Keywords: anti-inflammatory; glucocorticoid-induced leucine zipper; glucocorticoids; sepsis; sepsis-induced immunosuppression.
Copyright © 2020 He, Xu, Sun, Fang, Peng, Pan, Zhou, Wang and Shang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
[GILZ (glucocorticoid-induced leucine zipper), a mediator of the anti-inflammatory and immunosuppressive activity of glucocorticoids].Ann Ig. 2010 Jan-Feb;22(1 Suppl 1):53-9. Ann Ig. 2010. PMID: 20701225 Italian.
-
Role of GILZ in immune regulation, glucocorticoid actions and rheumatoid arthritis.Nat Rev Rheumatol. 2011 Jun;7(6):340-8. doi: 10.1038/nrrheum.2011.59. Epub 2011 May 10. Nat Rev Rheumatol. 2011. PMID: 21556028 Review.
-
Implicating the Role of GILZ in Glucocorticoid Modulation of T-Cell Activation.Front Immunol. 2019 Aug 7;10:1823. doi: 10.3389/fimmu.2019.01823. eCollection 2019. Front Immunol. 2019. PMID: 31440237 Free PMC article. Review.
-
Synthesis of glucocorticoid-induced leucine zipper (GILZ) by macrophages: an anti-inflammatory and immunosuppressive mechanism shared by glucocorticoids and IL-10.Blood. 2003 Jan 15;101(2):729-38. doi: 10.1182/blood-2002-02-0538. Epub 2002 Sep 12. Blood. 2003. PMID: 12393603
-
Glucocorticoids and Glucocorticoid-Induced-Leucine-Zipper (GILZ) in Psoriasis.Front Immunol. 2019 Sep 13;10:2220. doi: 10.3389/fimmu.2019.02220. eCollection 2019. Front Immunol. 2019. PMID: 31572404 Free PMC article. Review.
Cited by
-
Impact of duration of critical illness and level of systemic glucocorticoid availability on tissue-specific glucocorticoid receptor expression and actions: A prospective, observational, cross-sectional human and two translational mouse studies.EBioMedicine. 2022 Jun;80:104057. doi: 10.1016/j.ebiom.2022.104057. Epub 2022 May 15. EBioMedicine. 2022. PMID: 35584557 Free PMC article.
-
Glucocorticoid Resistance: Interference between the Glucocorticoid Receptor and the MAPK Signalling Pathways.Int J Mol Sci. 2021 Sep 17;22(18):10049. doi: 10.3390/ijms221810049. Int J Mol Sci. 2021. PMID: 34576214 Free PMC article. Review.
-
Immunomodulation by glucocorticoid-induced leucine zipper in macrophages: enhanced phagocytosis, protection from pyroptosis, and altered mitochondrial function.Front Immunol. 2024 May 23;15:1396827. doi: 10.3389/fimmu.2024.1396827. eCollection 2024. Front Immunol. 2024. PMID: 38855102 Free PMC article.
-
NLRC3 expression in macrophage impairs glycolysis and host immune defense by modulating the NF-κB-NFAT5 complex during septic immunosuppression.Mol Ther. 2023 Jan 4;31(1):154-173. doi: 10.1016/j.ymthe.2022.08.023. Epub 2022 Sep 6. Mol Ther. 2023. PMID: 36068919 Free PMC article.
-
Gender May Influence the Immunosuppressive Actions of Prednisone in Young Patients With Inflammatory Bowel Disease.Front Immunol. 2021 May 13;12:673068. doi: 10.3389/fimmu.2021.673068. eCollection 2021. Front Immunol. 2021. PMID: 34054855 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

