Effect of a Traditional Chinese Medicine Formula (CoTOL) on Serum Uric Acid and Intestinal Flora in Obese Hyperuricemic Mice Inoculated with Intestinal Bacteria
- PMID: 33424995
- PMCID: PMC7775141
- DOI: 10.1155/2020/8831937
Effect of a Traditional Chinese Medicine Formula (CoTOL) on Serum Uric Acid and Intestinal Flora in Obese Hyperuricemic Mice Inoculated with Intestinal Bacteria
Abstract
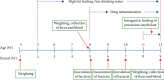
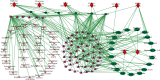
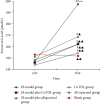
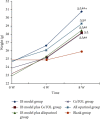
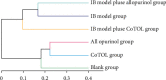
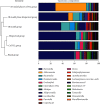
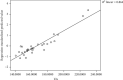
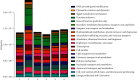
CoTOL is a traditional Chinese medicine (TCM) formula in clinics for treating gout and hyperuricemia, especially in obese patients with recurrent attacks. However, fewer studies have investigated how CoTOL impacts the intestinal flora in reducing uric acid. In the present, we analyze the bacteria targeted by ingredients of CoTOL and evaluate the effects of CoTOL on uric acid and intestinal flora in a mice model of obese hyperuricemia inoculated with xanthine dehydrogenase- (XOD-) producing bacteria, Streptococcus faecalis. Firstly, ingredients of herbs in CoTOL and gene target by these ingredients were retrieved from TCMID 2.0, and these genes were screened by DAVID Bioinformatics Resources 6.8, deciphered to retrieve the bacteria. Then, 3-4-week-old male C57bl/6j mice were randomly divided into 6 groups and fed with high fat diet for 8 weeks up to obesity standard. The mice were inoculated intragastrically with 5 × 109 CFU Streptococcus faecalis 3 times at the 5th, 6th, and 7th week and intragastrically administrated with uricase inhibitor, potassium-oxonate (PO, 250 mg/kg), to induce hyperuricemia at the 8th week, once a day for 7 consecutive days, respectively (IB model). IB model plus CoTOL (0.4 ml/20g) and allopurinol (40 mg/kg) were administrated by gavage at the 5th week, once a day for 4 weeks. The feces and blood in each group were sampled at the 4th and 8th week. With no bacteria inoculation, CoTOL, allopurinol, and blank group were treated with CoTOL and allopurinol or water, respectively. 44 species of bacteria (i.e., Enterococcus faecalis, Streptococcus, etc.) genes were targeted by 6 ingredients of 6 herbs in CoTOL. Inoculation with Streptococcus faecalis significantly caused the elevation of uric acid and the change of intestinal flora structure, whereas treatment with CoTOL significantly increased the abundance of Akkermansia and those of Bacteroides and Alloprevotella decreased. Furthermore, CoTOL exhibited a unique effect on reducing weight unobserved in allopurinol intervention. The present study, for the first time, demonstrated that CoTOL has beneficial effects on hyperuricemia and overweight, which may be attributed to regulating material metabolism and improving the structure or function of intestinal flora. Thus, CoTOL may be a promising therapy for hyperuricemia and overweight in chronic gout management and can be integrated with conventional treatments.
Copyright © 2020 Yan Gao et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Fenando A., Widrich J. Gout (podagra) StatPearls. 2020;5
LinkOut - more resources
Full Text Sources

