Transdermal Delivery Systems for Biomolecules
- PMID: 33425065
- PMCID: PMC7786146
- DOI: 10.1007/s12247-020-09525-2
Transdermal Delivery Systems for Biomolecules
Abstract
Purpose: The present review article focuses on highlighting the main technologies used as tools that improve the delivery of transdermal biomolecules, addressing them from the point of view of research in the development of transdermal systems that use physical and chemical permeation enhancers and nanocarrier systems or a combination of them.
Results: Transdermal drug delivery systems have increased in importance since the late 1970s when their use was approved by the Food and Drug Administration (FDA). They appeared to be an alternative resource for the administration of many potent drugs. The first transdermal drug delivery system used for biomolecules was for the treatment of hormonal disorders. Biomolecules have been used primarily in many treatments for cancer and diabetes, vaccines, hormonal disorders, and contraception.
Conclusions: The latest technologies that have used such transdermal biomolecule transporters include electrical methods (physical penetration enhancers), some chemical penetration enhancers and nanocarriers. All of them allow the maintenance of the physical and chemical properties of the main proteins and peptides through these clinical treatments, allowing their efficient storage, transport, and release and ensuring the achievement of their target and better results in the treatment of many diseases.
Keywords: Biomolecules; Chemical and physical enhancers; Microneedles; Proteins; Transdermal drug delivery; Transdermal systems.
© The Author(s), under exclusive licence to Springer Science+Business Media, LLC part of Springer Nature 2021.
Conflict of interest statement
Conflict of InterestThe authors declare no conflict of interest regarding the content and publication of this article.
Figures
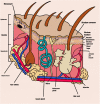
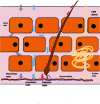

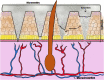
References
-
- Barbé C., Bartlett J., Kong L., Finnie K., Qiang Lin H., Larkin M., Calleja S., Bush and Calleja G. Silica particles: a novel drug-delivery system. Advanced Materials. 2004, 16, 21: 1959-1966.
-
- Teo AL, Shearwood C, Ng KC, Lu J, Moochhala S. Transdermal microneedles for drug delivery applications. Mater Sci Eng B. 2006;132(1–2):151–154. doi: 10.1016/j.mseb.2006.02.008. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
