Biomechanical factors in three-dimensional tissue bioprinting
- PMID: 33425087
- PMCID: PMC7780402
- DOI: 10.1063/5.0023206
Biomechanical factors in three-dimensional tissue bioprinting
Abstract
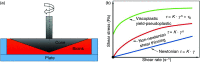
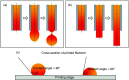
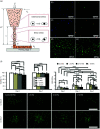
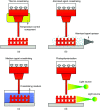
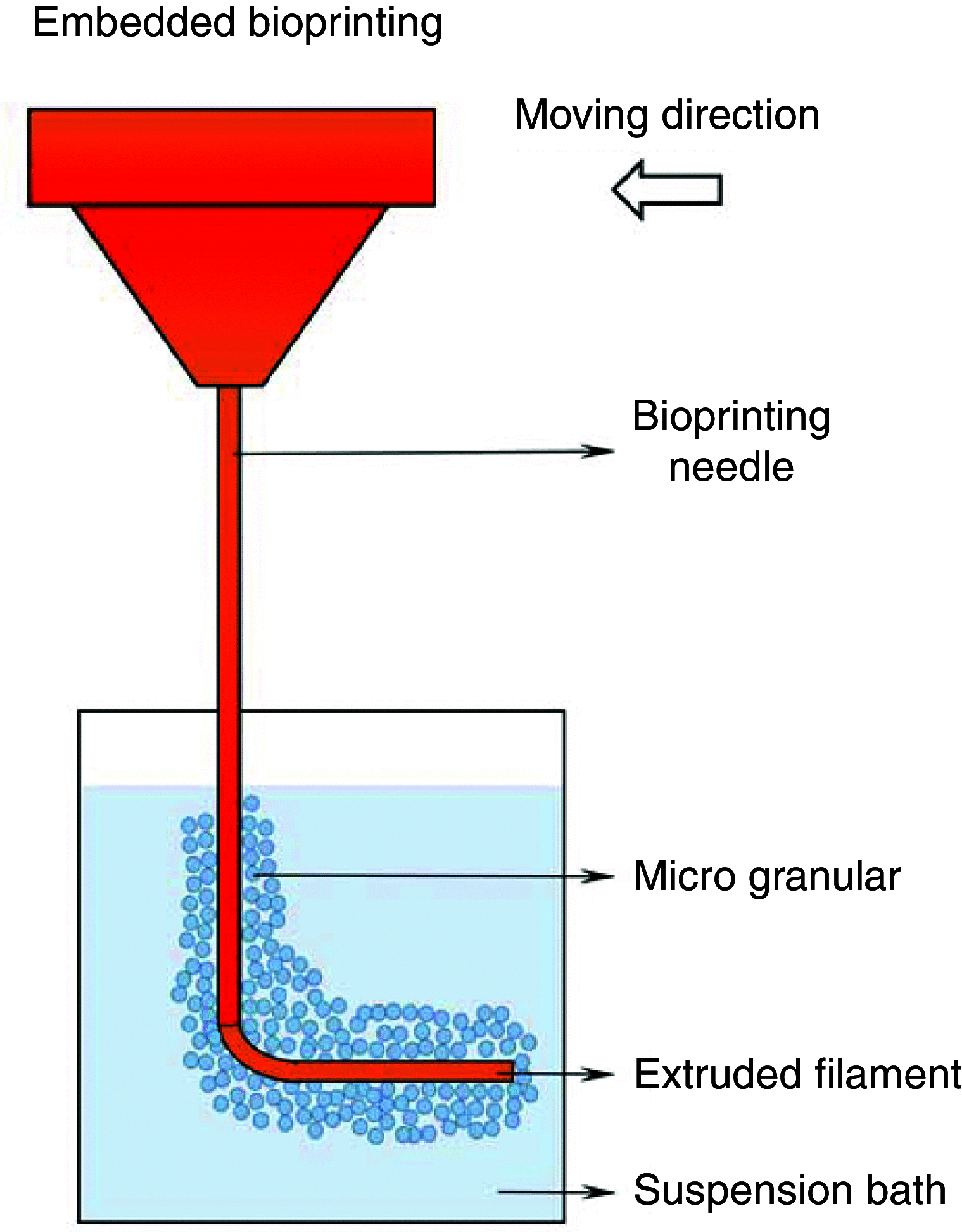
3D bioprinting techniques have shown great promise in various fields of tissue engineering and regenerative medicine. Yet, creating a tissue construct that faithfully represents the tightly regulated composition, microenvironment, and function of native tissues is still challenging. Among various factors, biomechanics of bioprinting processes play fundamental roles in determining the ultimate outcome of manufactured constructs. This review provides a comprehensive and detailed overview on various biomechanical factors involved in tissue bioprinting, including those involved in pre, during, and post printing procedures. In preprinting processes, factors including viscosity, osmotic pressure, and injectability are reviewed and their influence on cell behavior during the bioink preparation is discussed, providing a basic guidance for the selection and optimization of bioinks. In during bioprinting processes, we review the key characteristics that determine the success of tissue manufacturing, including the rheological properties and surface tension of the bioink, printing flow rate control, process-induced mechanical forces, and the in situ cross-linking mechanisms. Advanced bioprinting techniques, including embedded and multi-material printing, are explored. For post printing steps, general techniques and equipment that are used for characterizing the biomechanical properties of printed tissue constructs are reviewed. Furthermore, the biomechanical interactions between printed constructs and various tissue/cell types are elaborated for both in vitro and in vivo applications. The review is concluded with an outlook regarding the significance of biomechanical processes in tissue bioprinting, presenting future directions to address some of the key challenges faced by the bioprinting community.
Figures


Similar articles
-
Engineering considerations in the design of tissue specific bioink for 3D bioprinting applications.Biomater Sci. 2024 Dec 17;13(1):93-129. doi: 10.1039/d4bm01192a. Biomater Sci. 2024. PMID: 39535021 Review.
-
Alginate-Based Bioinks for 3D Bioprinting and Fabrication of Anatomically Accurate Bone Grafts.Tissue Eng Part A. 2021 Sep;27(17-18):1168-1181. doi: 10.1089/ten.TEA.2020.0305. Epub 2021 Feb 26. Tissue Eng Part A. 2021. PMID: 33218292 Free PMC article.
-
Nanocomposite bioinks for 3D bioprinting.Acta Biomater. 2022 Oct 1;151:45-69. doi: 10.1016/j.actbio.2022.08.014. Epub 2022 Aug 13. Acta Biomater. 2022. PMID: 35970479 Review.
-
Advancing bioinks for 3D bioprinting using reactive fillers: A review.Acta Biomater. 2020 Sep 1;113:1-22. doi: 10.1016/j.actbio.2020.06.040. Epub 2020 Jul 2. Acta Biomater. 2020. PMID: 32622053 Review.
-
Crosslinking Strategies for 3D Bioprinting of Polymeric Hydrogels.Small. 2020 Sep;16(35):e2002931. doi: 10.1002/smll.202002931. Epub 2020 Jul 30. Small. 2020. PMID: 32734720 Free PMC article. Review.
Cited by
-
Formulation and evaluation of a bioink composed of alginate, gelatin, and nanocellulose for meniscal tissue engineering.Int J Bioprint. 2022 Oct 14;9(1):621. doi: 10.18063/ijb.v9i1.621. eCollection 2023. Int J Bioprint. 2022. PMID: 36844246 Free PMC article.
-
A 3D Bioprinted in vitro Model of Neuroblastoma Recapitulates Dynamic Tumor-Endothelial Cell Interactions Contributing to Solid Tumor Aggressive Behavior.Adv Sci (Weinh). 2022 Aug;9(23):e2200244. doi: 10.1002/advs.202200244. Epub 2022 May 29. Adv Sci (Weinh). 2022. PMID: 35644929 Free PMC article.
-
Improving tumor microenvironment assessment in chip systems through next-generation technology integration.Front Bioeng Biotechnol. 2024 Sep 25;12:1462293. doi: 10.3389/fbioe.2024.1462293. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39386043 Free PMC article. Review.
-
Computer vision-aided bioprinting for bone research.Bone Res. 2022 Feb 25;10(1):21. doi: 10.1038/s41413-022-00192-2. Bone Res. 2022. PMID: 35217642 Free PMC article. Review.
-
Intelligent Vascularized 3D/4D/5D/6D-Printed Tissue Scaffolds.Nanomicro Lett. 2023 Oct 31;15(1):239. doi: 10.1007/s40820-023-01187-2. Nanomicro Lett. 2023. PMID: 37907770 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
