Pharmacogenomic information from CPIC and DPWG guidelines and its application on drug labels
- PMID: 33425802
- PMCID: PMC7781807
- DOI: 10.12793/tcp.2020.28.e18
Pharmacogenomic information from CPIC and DPWG guidelines and its application on drug labels
Abstract
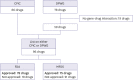
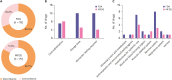
There are several hurdles to overcome before implementing pharmacogenomics (PGx) in precision medicine. One of the hurdles is unawareness of PGx by clinicians due to insufficient pharmacogenomic information on drug labels. Therefore, it might be important to implement PGx that reflects pharmacogenomic information on drug labels, standard of prescription for clinicians. This study aimed to evaluate the level at which PGx was being used in clinical practice by comparing the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group guidelines and drug labels of the US Food and Drug Administration (FDA) and the Korea Ministry of Food and Drug Safety (MFDS). Two PGx guidelines and drugs labels were scrutinized, and the concordance of the pharmacogenomic information between guidelines and drug labels was confirmed. The concordance of the label between FDA and MFDS was analyzed. In FDA labels, the number of concordant drug with guidelines was 24, while 13 drugs were concordant with MFDS labels. The number of drugs categorized as contraindication, change dose, and biomarker testing required was 7, 12 and 12 for the FDA and 8, 5 and 4 for the MFDS, respectively. The pharmacogenomic information of 9 drugs approved by both FDA and MFDS was identical. In conclusion, pharmacogenomic information on clinical implementation guidelines was limited on both FDA and MFDS labels because of various reasons including the characteristics of the guidelines and the drug labels. Therefore, more effort from pharmaceutical companies, academia and regulatory affairs needs to be made to implement pharmacogenomic information on drug labels.
Keywords: Drug Labeling; Guideline; Korea Ministry of Food and Drug Safety; Pharmacogenomics; United States Food and Drug Administration.
Copyright © 2020 Translational and Clinical Pharmacology.
Conflict of interest statement
Conflict of Interest: - Authors: Nothing to declare - Reviewers: Nothing to declare - Editors: Nothing to declare
Figures

Similar articles
-
ADME Gene-Related Pharmacogenomic Labeling of FDA-Approved Drugs: Comparison with Clinical Pharmacogenetics Implementation Consortium (CPIC) Evidence Levels.Medicines (Basel). 2024 Feb 20;11(3):6. doi: 10.3390/medicines11030006. Medicines (Basel). 2024. PMID: 38535119 Free PMC article.
-
Comparison of FDA Table of Pharmacogenetic Associations and Clinical Pharmacogenetics Implementation Consortium guidelines.Am J Health Syst Pharm. 2022 Jun 7;79(12):993-1005. doi: 10.1093/ajhp/zxac064. Am J Health Syst Pharm. 2022. PMID: 35230418 Free PMC article.
-
Evaluation of Current Regulation and Guidelines of Pharmacogenomic Drug Labels: Opportunities for Improvements.Clin Pharmacol Ther. 2020 May;107(5):1240-1255. doi: 10.1002/cpt.1720. Epub 2019 Dec 17. Clin Pharmacol Ther. 2020. PMID: 31715018 Free PMC article.
-
Unraveling heterogeneity of the clinical pharmacogenomic guidelines in oncology practice among major regulatory bodies.Pharmacogenomics. 2020 Nov;21(17):1247-1264. doi: 10.2217/pgs-2020-0056. Epub 2020 Oct 30. Pharmacogenomics. 2020. PMID: 33124490 Review.
-
Ubiquitous Pharmacogenomics (U-PGx): The Time for Implementation is Now. An Horizon2020 Program to Drive Pharmacogenomics into Clinical Practice.Curr Pharm Biotechnol. 2017;18(3):204-209. doi: 10.2174/1389201018666170103103619. Curr Pharm Biotechnol. 2017. PMID: 28044932 Review.
Cited by
-
Clinical pharmacogenomics in action: design, assessment and implementation of a novel pharmacogenetic panel supporting drug selection for diseases of the central nervous system (CNS).J Transl Med. 2021 Apr 15;19(1):151. doi: 10.1186/s12967-021-02816-3. J Transl Med. 2021. PMID: 33858454 Free PMC article.
-
Pharmaco-Multiomics: A New Frontier in Precision Psychiatry.Int J Mol Sci. 2025 Jan 26;26(3):1082. doi: 10.3390/ijms26031082. Int J Mol Sci. 2025. PMID: 39940850 Free PMC article. Review.
-
ADME Gene-Related Pharmacogenomic Labeling of FDA-Approved Drugs: Comparison with Clinical Pharmacogenetics Implementation Consortium (CPIC) Evidence Levels.Medicines (Basel). 2024 Feb 20;11(3):6. doi: 10.3390/medicines11030006. Medicines (Basel). 2024. PMID: 38535119 Free PMC article.
-
Comparison of FDA Table of Pharmacogenetic Associations and Clinical Pharmacogenetics Implementation Consortium guidelines.Am J Health Syst Pharm. 2022 Jun 7;79(12):993-1005. doi: 10.1093/ajhp/zxac064. Am J Health Syst Pharm. 2022. PMID: 35230418 Free PMC article.
-
Pharmacogenetic Guidelines for Psychotropic Drugs: Optimizing Prescriptions in Clinical Practice.Pharmaceutics. 2023 Oct 27;15(11):2540. doi: 10.3390/pharmaceutics15112540. Pharmaceutics. 2023. PMID: 38004520 Free PMC article. Review.
References
-
- Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med. 2015;372:2229–2234. - PubMed
-
- van der Wouden CH, Cambon-Thomsen A, Cecchin E, Cheung KC, Dávila-Fajardo CL, Deneer VH, et al. Implementing pharmacogenomics in Europe: design and implementation strategy of the ubiquitous pharmacogenomics consortium. Clin Pharmacol Ther. 2017;101:341–358. - PubMed
LinkOut - more resources
Full Text Sources
