hBMSC-Derived Extracellular Vesicles Attenuate IL-1β-Induced Catabolic Effects on OA-Chondrocytes by Regulating Pro-inflammatory Signaling Pathways
- PMID: 33425869
- PMCID: PMC7793861
- DOI: 10.3389/fbioe.2020.603598
hBMSC-Derived Extracellular Vesicles Attenuate IL-1β-Induced Catabolic Effects on OA-Chondrocytes by Regulating Pro-inflammatory Signaling Pathways
Abstract
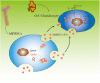
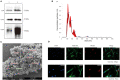
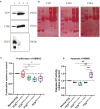
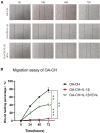
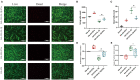
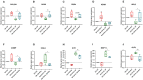
Background: Human bone marrow-derived mesenchymal stromal cells (hBMSCs) provide a promising therapeutic approach in the cell-based therapy of osteoarthritis (OA). However, several disadvantages evolved recently, including immune responses of the host and regulatory hurdles, making it necessary to search for alternative treatment options. Extracellular vesicles (EVs) are released by multiple cell types and tissues into the extracellular microenvironment, acting as message carriers during intercellular communication. Here, we investigate putative protective effects of hBMSC-derived EVs as a cell-free approach, on IL-1β-stimulated chondrocytes obtained from OA-patients. Methods: EVs were harvested from the cell culture supernatant of hBMSCs by a sequential ultracentrifugation process. Western blot, scanning electron microscopy (SEM), and nanoparticle tracking analysis (NTA) were performed to characterize the purified particles as EVs. Intracellular incorporation of EVs, derived from PHK26-labeled hBMSCs, was tested by adding the labeled EVs to human OA chondrocytes (OA-CH), followed by fluorescence microscopy. Chondrocytes were pre-stimulated with IL-1β for 24 h, followed by EVs treatment for 24 h. Subsequently, proliferation, apoptosis, and migration (wound healing) were analyzed via BrdU assay, caspase 3/7 assay, and scratch assay, respectively. With qRT-PCR, the relative expression level of anabolic and catabolic genes was determined. Furthermore, immunofluorescence microscopy and western blot were performed to evaluate the protein expression and phosphorylation levels of Erk1/2, PI3K/Akt, p38, TAK1, and NF-κB as components of pro-inflammatory signaling pathways in OA-CH. Results: EVs from hBMSCs (hBMSC-EVs) promote proliferation and reduce apoptosis of OA-CH and IL-1β-stimulated OA-CH. Moreover, hBMSC-EVs attenuate IL-1β-induced reduction of chondrocyte migration. Furthermore, hBMSC-EVs increase gene expression of PRG4, BCL2, and ACAN (aggrecan) and decrease gene expression of MMP13, ALPL, and IL1ß in OA-CH. Notably, COL2A1, SOX9, BCL2, ACAN, and COMP gene expression levels were significantly increased in IL-1β+ EV groups compared with those IL-1β groups without EVs, whereas the gene expression levels of COLX, IL1B, MMP13, and ALPL were significantly decreased in IL-1β+ EV groups compared to IL-1β groups without EVs. In addition, the phosphorylation status of Erk1/2, PI3K/Akt, p38, TAK1, and NF-κB signaling molecules, induced by IL-1β, is prevented by hBMSC- EVs. Conclusion: EVs derived from hBMSCs alleviated IL-1β-induced catabolic effects on OA-CH via promoting proliferation and migration and reducing apoptosis, probably via downregulation of IL-1ß-activated pro-inflammatory Erk1/2, PI3K/Akt, p38, TAK1, and NF-κB signaling pathways. EVs released from BMSCs may be considered as promising cell-free intervention strategy in cartilage regenerative medicine, avoiding several adverse effects of cell-based regenerative approaches.
Keywords: IL-1ß; chondrocytes; extracellular vesicles; hBMSC; osteoarthritis; signaling pathways.
Copyright © 2020 Li, Stöckl, Lukas, Götz, Herrmann, Federlin and Grässel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

