Multi-Path Optimization for Efficient Production of 2'-Fucosyllactose in an Engineered Escherichia coli C41 (DE3) Derivative
- PMID: 33425876
- PMCID: PMC7793955
- DOI: 10.3389/fbioe.2020.611900
Multi-Path Optimization for Efficient Production of 2'-Fucosyllactose in an Engineered Escherichia coli C41 (DE3) Derivative
Abstract
2'-fucosyllactose (2'-FL), one of the simplest but most abundant oligosaccharides in human milk, has been demonstrated to have many positive benefits for the healthy development of newborns. However, the high-cost production and limited availability restrict its widespread use in infant nutrition and further research on its potential functions. In this study, on the basis of previous achievements, we developed a powerful cell factory by using a lacZ-mutant Escherichia coli C41 (DE3)ΔZ to ulteriorly increase 2'-FL production by feeding inexpensive glycerol. Initially, we co-expressed the genes for GDP-L-fucose biosynthesis and heterologous α-1,2-fucosyltransferase in C41(DE3)ΔZ through different plasmid-based expression combinations, functionally constructing a preferred route for 2'-FL biosynthesis. To further boost the carbon flux from GDP-L-fucose toward 2'-FL synthesis, deletion of chromosomal genes (wcaJ, nudD, and nudK) involved in the degradation of the precursors GDP-L-fucose and GDP-mannose were performed. Notably, the co-introduction of two heterologous positive regulators, RcsA and RcsB, was confirmed to be more conducive to GDP-L-fucose formation and thus 2'-FL production. Further a genomic integration of an individual copy of α-1,2-fucosyltransferase gene, as well as the preliminary optimization of fermentation conditions enabled the resulting engineered strain to achieve a high titer and yield. By collectively taking into account the intracellular lactose utilization, GDP-L-fucose availability, and fucosylation activity for 2'-FL production, ultimately a highest titer of 2'-FL in our optimized conditions reached 6.86 g/L with a yield of 0.92 mol/mol from lactose in the batch fermentation. Moreover, the feasibility of mass production was demonstrated in a 50-L fed-batch fermentation system in which a maximum titer of 66.80 g/L 2'-FL was achieved with a yield of 0.89 mol 2'-FL/mol lactose and a productivity of approximately 0.95 g/L/h 2'-FL. As a proof of concept, our preliminary 2'-FL production demonstrated a superior production performance, which will provide a promising candidate process for further industrial production.
Keywords: 2′-fucosyllactose; Escherichia coli; GDP-D-mannose; GDP-L-fucose; fucosylation.
Copyright © 2020 Ni, Li, Wu, Ge, Liao, Yuan, Chen and Yao.
Conflict of interest statement
JW, LY, and XC were employed by the company Wuhan Zhongke Optics Valley Green Biotechnology Co. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
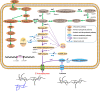
 ), glycerol (
), glycerol ( ), 2′-FL (
), 2′-FL ( ), and DCW (
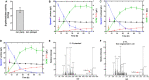
), and DCW ( ). Brown vertical arrow indicates the time point for IPTG induction. Purple vertical arrow indicates the time point for lactose addition. (E,F) LC/MS identification of 2′-FL produced by the recombinant E. coli C41 (DE3)ΔZ. The red arrows represent the 2′-FL debris from the mass spectrometer.
). Brown vertical arrow indicates the time point for IPTG induction. Purple vertical arrow indicates the time point for lactose addition. (E,F) LC/MS identification of 2′-FL produced by the recombinant E. coli C41 (DE3)ΔZ. The red arrows represent the 2′-FL debris from the mass spectrometer.
 ), glycerol (
), glycerol ( ), 2′-FL (
), 2′-FL ( ), and DCW (
), and DCW ( ). Brown vertical arrow indicates the time point for IPTG induction. Purple vertical arrow indicates the time point for lactose addition.
). Brown vertical arrow indicates the time point for IPTG induction. Purple vertical arrow indicates the time point for lactose addition.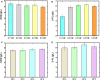
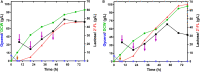
 ), glycerol (
), glycerol ( ), 2′-FL (
), 2′-FL ( ), and DCW (
), and DCW ( ). Brown vertical arrow indicates the time point for IPTG induction. Purple vertical arrow indicates the time point for lactose addition.
). Brown vertical arrow indicates the time point for IPTG induction. Purple vertical arrow indicates the time point for lactose addition.References
-
- Abbott Laboratories. (2020). Similac. Available online at: https://similac.com/toddler-formula/go-grow-toddler-immune-support?utm_c... (accessed July 15, 2020).
-
- Bhattacharya S. K., Dubey A. K. (1995). Metabolic burden as reflected by maintenance coefficient of recombinant Escherichia coli overexpressing target gene. Biotechnol. Lett. 17, 1155–1160. 10.1007/BF00128377 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

