Influence of Extracellular Vesicles Isolated From Osteoblasts of Patients With Cox-Arthrosis and/or Osteoporosis on Metabolism and Osteogenic Differentiation of BMSCs
- PMID: 33425878
- PMCID: PMC7785908
- DOI: 10.3389/fbioe.2020.615520
Influence of Extracellular Vesicles Isolated From Osteoblasts of Patients With Cox-Arthrosis and/or Osteoporosis on Metabolism and Osteogenic Differentiation of BMSCs
Abstract
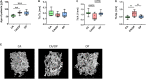
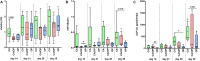
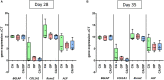
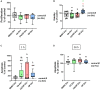
Background: Studies with extracellular vesicles (EVs), including exosomes, isolated from mesenchymal stem cells (MSC) indicate benefits for the treatment of musculoskeletal pathologies as osteoarthritis (OA) and osteoporosis (OP). However, little is known about intercellular effects of EVs derived from pathologically altered cells that might influence the outcome by counteracting effects from "healthy" MSC derived EVs. We hypothesize, that EVs isolated from osteoblasts of patients with hip OA (coxarthrosis/CA), osteoporosis (OP), or a combination of both (CA/OP) might negatively affect metabolism and osteogenic differentiation of bone-marrow derived (B)MSCs. Methods: Osteoblasts, isolated from bone explants of CA, OP, and CA/OP patients, were compared regarding growth, viability, and osteogenic differentiation capacity. Structural features of bone explants were analyzed via μCT. EVs were isolated from supernatant of naïve BMSCs and CA, OP, and CA/OP osteoblasts (osteogenic culture for 35 days). BMSC cultures were stimulated with EVs and subsequently, cell metabolism, osteogenic marker gene expression, and osteogenic differentiation were analyzed. Results: Trabecular bone structure was different between the three groups with lowest number and highest separation in the CA/OP group. Viability and Alizarin red staining increased over culture time in CA/OP osteoblasts whereas growth of osteoblasts was comparable. Alizarin red staining was by trend higher in CA compared to OP osteoblasts after 35 days and ALP activity was higher after 28 and 35 days. Stimulation of BMSC cultures with CA, OP, and CA/OP EVs did not affect proliferation but increased caspase 3/7-activity compared to unstimulated BMSCs. BMSC viability was reduced after stimulation with CA and CA/OP EVs compared to unstimulated BMSCs or stimulation with OP EVs. ALP gene expression and activity were reduced in BMSCs after stimulation with CA, OP, and CA/OP EVs. Stimulation of BMSCs with CA EVs reduced Alizarin Red staining by trend. Conclusion: Stimulation of BMSCs with EVs isolated from CA, OP, and CA/OP osteoblasts had mostly catabolic effects on cell metabolism and osteogenic differentiation irrespective of donor pathology and reflect the impact of tissue microenvironment on cell metabolism. These catabolic effects are important for understanding differences in effects of EVs on target tissues/cells when harnessing them as therapeutic drugs.
Keywords: EVs; extracellular vesicles; mesenchymal stem cells; osteoarthritis; osteoblasts; osteogenic differentiation; osteoporosis.
Copyright © 2020 Niedermair, Lukas, Li, Stöckl, Craiovan, Brochhausen, Federlin, Herrmann and Grässel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Extracellular vesicles from GPNMB-modified bone marrow mesenchymal stem cells attenuate bone loss in an ovariectomized rat model.Life Sci. 2021 May 1;272:119208. doi: 10.1016/j.lfs.2021.119208. Epub 2021 Feb 11. Life Sci. 2021. PMID: 33582177
-
ASPN Synergizes with HAPLN1 to Inhibit the Osteogenic Differentiation of Bone Marrow Mesenchymal Stromal Cells and Extracellular Matrix Mineralization of Osteoblasts.Orthop Surg. 2023 Sep;15(9):2423-2434. doi: 10.1111/os.13803. Epub 2023 Jul 10. Orthop Surg. 2023. PMID: 37427673 Free PMC article.
-
Human umbilical cord mesenchymal stromal cells-derived extracellular vesicles exert potent bone protective effects by CLEC11A-mediated regulation of bone metabolism.Theranostics. 2020 Jan 16;10(5):2293-2308. doi: 10.7150/thno.39238. eCollection 2020. Theranostics. 2020. PMID: 32089743 Free PMC article.
-
Application and Molecular Mechanisms of Extracellular Vesicles Derived from Mesenchymal Stem Cells in Osteoporosis.Curr Issues Mol Biol. 2022 Dec 15;44(12):6346-6367. doi: 10.3390/cimb44120433. Curr Issues Mol Biol. 2022. PMID: 36547094 Free PMC article. Review.
-
Enhancing osteoporosis treatment with engineered mesenchymal stem cell-derived extracellular vesicles: mechanisms and advances.Cell Death Dis. 2024 Feb 8;15(2):119. doi: 10.1038/s41419-024-06508-w. Cell Death Dis. 2024. PMID: 38331884 Free PMC article. Review.
Cited by
-
The Research Progress of Exosomes in Osteoarthritis, With Particular Emphasis on the Mediating Roles of miRNAs and lncRNAs.Front Pharmacol. 2021 May 21;12:685623. doi: 10.3389/fphar.2021.685623. eCollection 2021. Front Pharmacol. 2021. PMID: 34093208 Free PMC article. Review.
-
Osteoblast Differentiation and Signaling: Established Concepts and Emerging Topics.Int J Mol Sci. 2021 Jun 22;22(13):6651. doi: 10.3390/ijms22136651. Int J Mol Sci. 2021. PMID: 34206294 Free PMC article. Review.
-
Targeting osteoarthritis with small extracellular vesicle therapy: potential and perspectives.Front Bioeng Biotechnol. 2025 Jun 20;13:1570526. doi: 10.3389/fbioe.2025.1570526. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40621210 Free PMC article.
-
Delivery of Growth Factors to Enhance Bone Repair.Bioengineering (Basel). 2023 Oct 26;10(11):1252. doi: 10.3390/bioengineering10111252. Bioengineering (Basel). 2023. PMID: 38002376 Free PMC article. Review.
-
Total Flavonoids from Rhizoma Drynariae (Gusuibu) Alleviates Diabetic Osteoporosis by Activating BMP2/Smad Signaling Pathway.Comb Chem High Throughput Screen. 2023;26(13):2401-2409. doi: 10.2174/1386207326666230223165730. Comb Chem High Throughput Screen. 2023. PMID: 36825725 Free PMC article.
References
-
- Brady J. V., Troyer R. M., Ramsey S. A., Leeper H., Yang L., Maier C. S., et al. (2018). A preliminary proteomic investigation of circulating exosomes and discovery of biomarkers associated with the progression of Osteosarcoma in a clinical model of spontaneous disease. Transl. Oncol. 11 1137–1146. 10.1016/j.tranon.2018.07.004 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

