The Ocular Neural Crest: Specification, Migration, and Then What?
- PMID: 33425902
- PMCID: PMC7785809
- DOI: 10.3389/fcell.2020.595896
The Ocular Neural Crest: Specification, Migration, and Then What?
Abstract
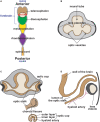
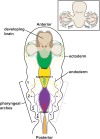
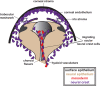
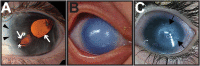
During vertebrate embryonic development, a population of dorsal neural tube-derived stem cells, termed the neural crest (NC), undergo a series of morphogenetic changes and extensive migration to become a diverse array of cell types. Around the developing eye, this multipotent ocular NC cell population, called the periocular mesenchyme (POM), comprises migratory mesenchymal cells that eventually give rise to many of the elements in the anterior of the eye, such as the cornea, sclera, trabecular meshwork, and iris. Molecular cell biology and genetic analyses of congenital eye diseases have provided important information on the regulation of NC contributions to this area of the eye. Nevertheless, a complete understanding of the NC as a contributor to ocular development remains elusive. In addition, positional information during ocular NC migration and the molecular pathways that regulate end tissue differentiation have yet to be fully elucidated. Further, the clinical challenges of ocular diseases, such as Axenfeld-Rieger syndrome (ARS), Peters anomaly (PA) and primary congenital glaucoma (PCG), strongly suggest the need for better treatments. While several aspects of NC evolution have recently been reviewed, this discussion will consolidate the most recent current knowledge on the specification, migration, and contributions of the NC to ocular development, highlighting the anterior segment and the knowledge obtained from the clinical manifestations of its associated diseases. Ultimately, this knowledge can inform translational discoveries with potential for sorely needed regenerative therapies.
Keywords: anterior segment; neural crest; ocular development; ocular diseases; periocular mesenchyme.
Copyright © 2020 Williams and Bohnsack.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Baulmann D. C., Ohlmann A., Flugel-Koch C., Goswami S., Cvekl A., Tamm E. R. (2002). Pax6 heterozygous eyes show defects in chamber angle differentiation that are associated with a wide spectrum of other anterior eye segment abnormalities. Mech. Dev. 118 3–17. 10.1016/s0925-4773(02)00260-5 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

