SESTRINs: Emerging Dynamic Stress-Sensors in Metabolic and Environmental Health
- PMID: 33425907
- PMCID: PMC7794007
- DOI: 10.3389/fcell.2020.603421
SESTRINs: Emerging Dynamic Stress-Sensors in Metabolic and Environmental Health
Abstract
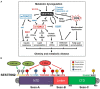
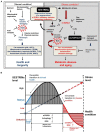
Proper timely management of various external and internal stresses is critical for metabolic and redox homeostasis in mammals. In particular, dysregulation of mechanistic target of rapamycin complex (mTORC) triggered from metabolic stress and accumulation of reactive oxygen species (ROS) generated from environmental and genotoxic stress are well-known culprits leading to chronic metabolic disease conditions in humans. Sestrins are one of the metabolic and environmental stress-responsive groups of proteins, which solely have the ability to regulate both mTORC activity and ROS levels in cells, tissues and organs. While Sestrins are originally reported as one of several p53 target genes, recent studies have further delineated the roles of this group of stress-sensing proteins in the regulation of insulin sensitivity, glucose and fat metabolism, and redox-function in metabolic disease and aging. In this review, we discuss recent studies that investigated and manipulated Sestrins-mediated stress signaling pathways in metabolic and environmental health. Sestrins as an emerging dynamic group of stress-sensor proteins are drawing a spotlight as a preventive or therapeutic mechanism in both metabolic stress-associated pathologies and aging processes at the same time.
Keywords: ROS; Sestrins; aging; cancer; environmental stress; mTORC; metabolic disease; obesity/inflammation.
Copyright © 2020 Ro, Fay, Cyuzuzo, Jang, Lee, Song and Harris.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

