Lack of FAM20A, Ectopic Gingival Mineralization and Chondro/Osteogenic Modifications in Enamel Renal Syndrome
- PMID: 33425910
- PMCID: PMC7793853
- DOI: 10.3389/fcell.2020.605084
Lack of FAM20A, Ectopic Gingival Mineralization and Chondro/Osteogenic Modifications in Enamel Renal Syndrome
Abstract
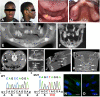
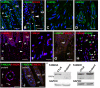
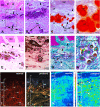
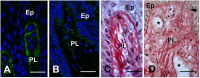
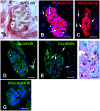
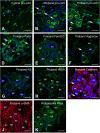
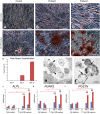
Enamel renal syndrome (ERS) is a rare recessive disorder caused by loss-of-function mutations in FAM20A (family with sequence similarity 20 member A, OMIM #611062). Enamel renal syndrome is characterized by amelogenesis imperfecta, delayed or failed tooth eruption, intrapulpal calcifications, gingival overgrowth and nephrocalcinosis. Although gingival overgrowth has consistently been associated with heterotopic calcifications the pathogenesis, structure and interactions of the mineral deposits with the surrounding connective tissue are largely unknown. We here report a novel FAM20A mutation in exon 1 (c.358C > T) introducing a premature stop codon (p.Gln120*) and resulting in a complete loss of FAM20A. In addition to the typical oral findings and nephrocalcinosis, ectopic calcified nodules were also seen in the cervical and thoracic vertebrae regions. Histopathologic analysis of the gingiva showed an enlarged papillary layer associated with aberrant angiogenesis and a lamina propria displaying significant changes in its extracellular matrix composition, including disruption of the collagen I fiber network. Ectopic calcifications were found throughout the connective gingival tissue. Immunomorphological and ultrastructural analyses indicated that the calcification process was associated with epithelial degeneration and transformation of the gingival fibroblasts to chondro/osteoblastic-like cells. Mutant gingival fibroblasts cultures were prone to calcify and abnormally expressed osteoblastic markers such as RUNX2 or PERIOSTIN. Our findings expand the previously reported phenotypes and highlight some aspects of ERS pathogenesis.
Keywords: Fam20A; calcification; cell death; enamel renal syndrome; fibroblast; gingiva; osteoblast.
Copyright © 2020 Simancas Escorcia, Diarra, Naveau, Dessombz, Felizardo, Cannaya, Chatziantoniou, Quentric, Vikkula, Cases, Berdal, De La Dure-Molla and Kozyraki.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

