Purification and Functional Characterization of the Chloroform/Methanol-Soluble Protein 3 (CM3) From Triticum aestivum in Drosophila melanogaster
- PMID: 33425975
- PMCID: PMC7785803
- DOI: 10.3389/fnut.2020.607937
Purification and Functional Characterization of the Chloroform/Methanol-Soluble Protein 3 (CM3) From Triticum aestivum in Drosophila melanogaster
Abstract
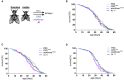
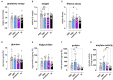
Non-celiac wheat sensitivity (NCWS) has been proposed to be an independent disease entity that is characterized by intestinal (e.g., abdominal pain, flatulence) and extra-intestinal symptoms (e.g., headache, fatigue), which are propagated following the ingestion of wheat products. Increased activity of amylase trypsin inhibitors (ATIs) in modern wheat is suggested to be major trigger of NCWS, while underlying mechanisms still remain elusive. Here, we aimed to generate and functionally characterize the most abundant ATI in modern wheat, chloroform/methanol-soluble protein 3 (CM3), in vitro and in Drosophila melanogaster. We demonstrate that CM3 displays α-glucosidase but not α-amylase or trypsin inhibitory activity in vitro. Moreover, fruit flies fed a sucrose-containing diet together with CM3 displayed significant overgrowth of intestinal bacteria in a sucrose-dependent manner while the consumption of α-amylase and α-glucosidase inhibitors was sufficient to limit bacterial quantities in the intestine. Notably, both CM3 and acarbose-treated flies showed a reduced lifespan. However, this effect was absent in amylase inhibitor (AI) treated flies. Together, given α-glucosidase is a crucial requirement for disaccharide digestion, we suggest that inhibition of α-glucosidase by CM3 enhances disaccharide load in the distal gastrointestinal tract, thereby promoting intestinal bacteria overgrowth. However, it remains speculative if this here described former unknown function of CM3 might contribute to the development of gastrointestinal symptoms observed in NCWS patients which are very similar to symptoms of patients with small intestinal bacterial overgrowth.
Keywords: ATIs; CM3; Drosophila melanogaster; non-celiac wheat sensitivity; α-glucosidase.
Copyright © 2020 Thiel, Ragab, Wagner, Divanovic, Derer and Sina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Nutritional Wheat Amylase-Trypsin Inhibitors Promote Intestinal Inflammation via Activation of Myeloid Cells.Gastroenterology. 2017 Apr;152(5):1100-1113.e12. doi: 10.1053/j.gastro.2016.12.006. Epub 2016 Dec 16. Gastroenterology. 2017. PMID: 27993525
-
Lactobacilli Degrade Wheat Amylase Trypsin Inhibitors to Reduce Intestinal Dysfunction Induced by Immunogenic Wheat Proteins.Gastroenterology. 2019 Jun;156(8):2266-2280. doi: 10.1053/j.gastro.2019.02.028. Epub 2019 Feb 22. Gastroenterology. 2019. PMID: 30802444
-
Breeding from 1891 to 2010 did not increase the content of amylase/trypsin-inhibitors in wheat (Triticum aestivum).NPJ Sci Food. 2023 Aug 23;7(1):43. doi: 10.1038/s41538-023-00219-w. NPJ Sci Food. 2023. PMID: 37612428 Free PMC article.
-
Non-celiac wheat sensitivity: differential diagnosis, triggers and implications.Best Pract Res Clin Gastroenterol. 2015 Jun;29(3):469-76. doi: 10.1016/j.bpg.2015.04.002. Epub 2015 May 8. Best Pract Res Clin Gastroenterol. 2015. PMID: 26060111 Review.
-
Amylase-Trypsin Inhibitors in Wheat and Other Cereals as Potential Activators of the Effects of Nonceliac Gluten Sensitivity.J Med Food. 2018 Mar;21(3):207-214. doi: 10.1089/jmf.2017.0018. Epub 2018 Jan 9. J Med Food. 2018. PMID: 29315017 Review.
Cited by
-
Testing the evidence that lifespan-extending compound interventions are conserved across laboratory animal model species.Geroscience. 2023 Jun;45(3):1401-1409. doi: 10.1007/s11357-022-00722-0. Epub 2023 Jan 13. Geroscience. 2023. PMID: 36637786 Free PMC article. Review.
-
Food supplementation with wheat gluten leads to climbing performance decline in Drosophila melanogaster.MicroPubl Biol. 2022 Sep 23;2022:10.17912/micropub.biology.000642. doi: 10.17912/micropub.biology.000642. eCollection 2022. MicroPubl Biol. 2022. PMID: 36217442 Free PMC article.
-
Structure, Function and Protein Engineering of Cereal-Type Inhibitors Acting on Amylolytic Enzymes.Front Mol Biosci. 2022 Mar 25;9:868568. doi: 10.3389/fmolb.2022.868568. eCollection 2022. Front Mol Biosci. 2022. PMID: 35402513 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

